Chemistry Notes - Unit Conversion
ADVERTISEMENT
Year 12 Chemistry Notes
Unit Conversion 1/2
Unit Conversion
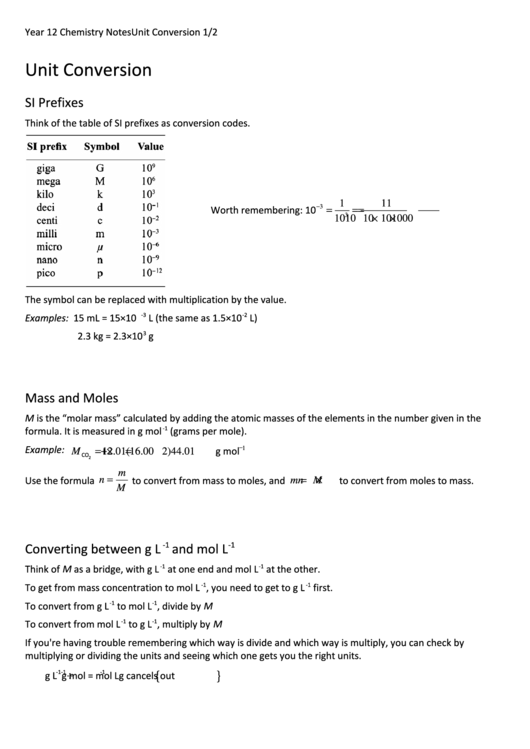
SI Prefixes
Think of the table of SI prefixes as conversion codes.
1
1
1
−
3
Worth remembering: 10
=
=
=
3
10
10 10 10
×
×
1000
The symbol can be replaced with multiplication by the value.
-3
-2
Examples: 15 mL = 15×10
L (the same as 1.5×10
L)
3
2.3 kg = 2.3×10
g
Mass and Moles
M is the “molar mass” calculated by adding the atomic masses of the elements in the number given in the
-1
formula. It is measured in g mol
(grams per mole).
−
1
Example:
g mol
M
=
12.01 (16.00 2) 44.01
+
×
=
CO
2
m
n
=
to convert from mass to moles, and m n M
= ×
Use the formula
to convert from moles to mass.
M
-1
-1
Converting between g L
and mol L
-1
-1
Think of M as a bridge, with g L
at one end and mol L
at the other.
-1
-1
To get from mass concentration to mol L
, you need to get to g L
first.
-1
-1
To convert from g L
to mol L
, divide by M
-1
-1
To convert from mol L
to g L
, multiply by M
If you're having trouble remembering which way is divide and which way is multiply, you can check by
multiplying or dividing the units and seeing which one gets you the right units.
-1
-1
-1
e.g.
{
}
g L
g mol = mol L
g cancels out
÷
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2