Chemistry Notes - Unit Conversion Page 2
ADVERTISEMENT
Year 12 Chemistry Notes
Unit Conversion 2/2
Same Unit Conversion
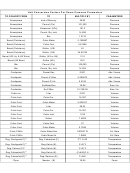
To convert a value to a different prefix but the same base unit, complete this equation:
1 old unit = ? new units
-1
-1
If you're converting the first part of the unit (for example the g in gL
or the mg in mg mol
)
Multiply the original value by the number found above to get the value in the new units.
-1
-1
Example: Convert 5 kg L
into g L
1 kg = 1000 g
-1
∴ 5×1000 = 5000 g L
-1
-1
Or, if you're converting the second part (the 'per' part, for example the L in g L
or the mol in mg mol
)
Divide the original value by the number found above to get the value in the new units.
-1
-1
Example: Convert 5 g L
into g (mL)
(from grams per litre into grams per millilitre)
1 L = 1000 mL
-1
∴ 5÷1000 = 0.005 g (mL)
Other things to Remember
-1
%w/v is the same as g (100mL)
(grams per hundred millilitres)
-1
%w/w is the same as g (100g)
(grams per hundred grams)
-1
-1
ppm is the same as mg L
or mg kg
, depending on whether the sample is dissolved or solid.
-1
-1
ppb is the same as µg L
or µg kg
, depending on whether the sample is dissolved or solid.
mass
Given a mass and a volume, the concentration can be calculated with
and the units will be in
C
=
volume
mass per volume, whatever units were used in the calculation.
-1
Example: 5 g in 1 L is 5 g L
moles
Given a number of moles and a volume, the same rule applies except with
and units of moles
C
=
volume
instead of units of mass.
You can also rearrange these formulas to convert from a concentration into a volume, number of moles, or
mass.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2