Empirical Formulas
ADVERTISEMENT
6.2H Empirical Formulas.doc
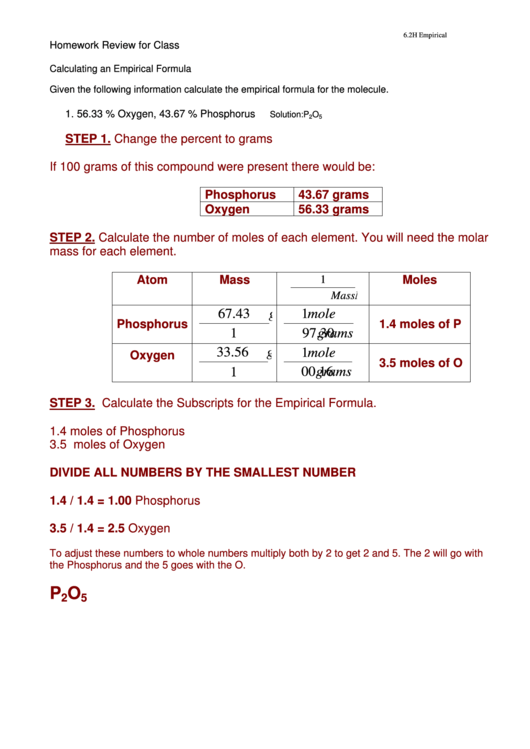
Homework Review for Class
Calculating an Empirical Formula
Given the following information calculate the empirical formula for the molecule.
1. 56.33 % Oxygen, 43.67 % Phosphorus
Solution:P
O
2
5
STEP 1. Change the percent to grams
If 100 grams of this compound were present there would be:
Phosphorus
43.67 grams
Oxygen
56.33 grams
STEP 2. Calculate the number of moles of each element. You will need the molar
mass for each element.
Atom
Mass
Moles
1
Molar _
Mass
43
.
67
grams
1
mole
Phosphorus
1.4 moles of P
1
30
.
97
grams
56
.
33
grams
1
mole
Oxygen
3.5 moles of O
1
16
.
00
grams
STEP 3. Calculate the Subscripts for the Empirical Formula.
1.4 moles of Phosphorus
3.5 moles of Oxygen
DIVIDE ALL NUMBERS BY THE SMALLEST NUMBER
1.4 / 1.4 = 1.00 Phosphorus
3.5 / 1.4 = 2.5
Oxygen
To adjust these numbers to whole numbers multiply both by 2 to get 2 and 5. The 2 will go with
the Phosphorus and the 5 goes with the O.
P
O
2
5
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4








