Quantum Numbers, N, L, Ml, Ms , And Electron Configuration Summary
ADVERTISEMENT
Quantum Numbers, n, l, m l , m s , and Electron Configuration Summary
Energy Level or Shell: n , or the Principle Quantum number: distance from the nucleus:
n = 1, 2, 3, 4, 5, 6, 7 ...
higher n is farther from the nucleus
Sublevel or Subshell: s, p, d, f or the Azimuthal quantum number: l = 0, 1, 2, 3.... (l = 0 to n–1)
describes the shape of orbital: s = spherical, p is dumbell, d is double dumbell
Orbital: Magnetic quantum number, m l , =– l to l : Orientation in space of the atomic orbital:
Each type of sublevel (or shape) has different numbers of orbitals
s has 1 orbital p has 3 orbitals: p x , p y , p z
d has 5 orbitals
f has 7 orbitals
Electron: Spin quantum number, m s = +1/2 or –1/2 : Which electron in the orbital?
There can be at most 2 electrons, with opposite spins, in any orbital.
s has 2 electrons
p has 6 electrons
d has 10 electrons
f has 14 electrons
Summary: In an Energy Level: There are n sublevels, n 2 orbitals, and 2n 2 maximum electrons
n
sublevel m l
n
l sublevel
m l
n
l sublevel
m l
l
1
0
1s
0
3
0
3s
0
4
0
4s
0
2
0
2s
0
1
3p
–1, 0, 1
1
4p
–1, 0, 1
1
2p
–1, 0, 1
2
3d
–2, –1, 0, 1, 2
2
4d
-2, -1, 0, 1, 2
3
4f
-3, -2, -1, 0, 1, 2, 3
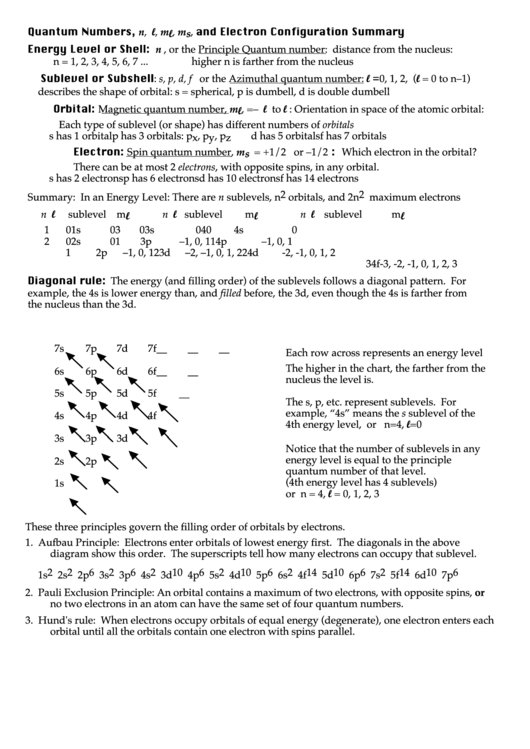
Diagonal rule: The energy (and filling order) of the sublevels follows a diagonal pattern. For
example, the 4s is lower energy than, and filled before, the 3d, even though the 4s is farther from
the nucleus than the 3d.
7s
7p
7d
7f
__
__
__
Each row across represents an energy level
The higher in the chart, the farther from the
6s
6p
6d
6f
__
__
nucleus the level is.
5s
5p
5d
5f
__
The s, p, etc. represent sublevels. For
example, “4s” means the s sublevel of the
4s
4p
4d
4f
4th energy level, or n=4, l=0
3s
3p
3d
Notice that the number of sublevels in any
energy level is equal to the principle
2s
2p
quantum number of that level.
(4th energy level has 4 sublevels)
1s
or n = 4, l = 0, 1, 2, 3
These three principles govern the filling order of orbitals by electrons.
1. Aufbau Principle: Electrons enter orbitals of lowest energy first. The diagonals in the above
diagram show this order. The superscripts tell how many electrons can occupy that sublevel.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6 ....
2. Pauli Exclusion Principle: An orbital contains a maximum of two electrons, with opposite spins, or
no two electrons in an atom can have the same set of four quantum numbers.
3. Hund's rule: When electrons occupy orbitals of equal energy (degenerate), one electron enters each
orbital until all the orbitals contain one electron with spins parallel.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








