Electron Configurations - Periodicity Questions And Answers
ADVERTISEMENT
South Pasadena AP Chemistry
8 Electron Configurations & Periodicity
A N S W E R S T O S T U D Y Q U E S T I O N S
1.
Write the electron configurations of the following elements using the shorthand notation for the noble
gas cores.
2
3
a. phosphorus
[Ne] 3s
3p
8
2
b. nickel
[Ar] 3d
4s
14
6
2
c. osmium
[Xe] 4f
5d
6s
10
2
d. californium
[Rn] 5f
7s
2
2
e. titanium
[Ar] 3d
4s
2.
Which orbital is filled following these orbitals?
a. 3d
is followed by 4p
b. 4s
is followed by 3d
c. 5p
is followed by 6s
d. 5f
is followed by 6d
3.
How many electrons can be accommodated in
a. a d subshell
10; two in each of 5 orbitals
b. a set of f orbitals
14; two in each of 7 orbitals
2
c. the n = 4 shell
32; 2n
d. the 7s orbital
2
e. a p
orbital?
2
x
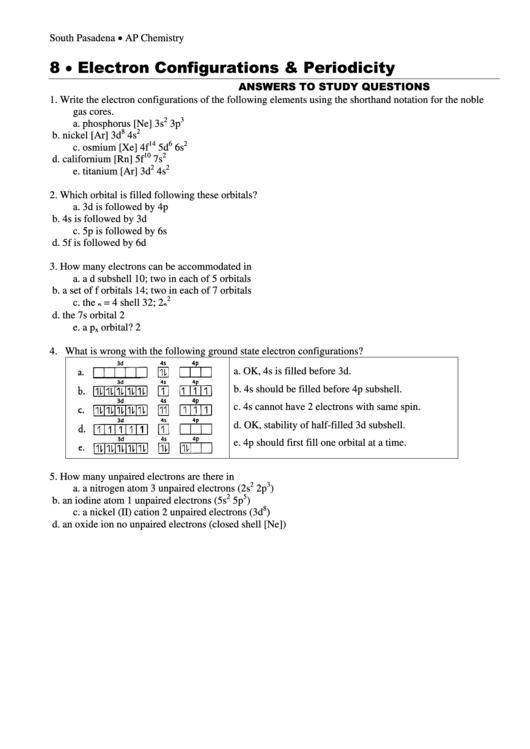
4.
What is wrong with the following ground state electron configurations?
a.
OK, 4s is filled before 3d.
b.
4s should be filled before 4p subshell.
c.
4s cannot have 2 electrons with same spin.
d.
OK, stability of half-filled 3d subshell.
e.
4p should first fill one orbital at a time.
5.
How many unpaired electrons are there in
2
3
a. a nitrogen atom
3 unpaired electrons
(2s
2p
)
2
5
b. an iodine atom
1 unpaired electrons
(5s
5p
)
8
c. a nickel (II) cation
2 unpaired electrons
(3d
)
d. an oxide ion
no unpaired electrons
(closed shell [Ne])
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3








