Geometric Isomerism
ADVERTISEMENT
C h e m g u i d e – a n s w e r s
GEOMETRIC ISOMERISM
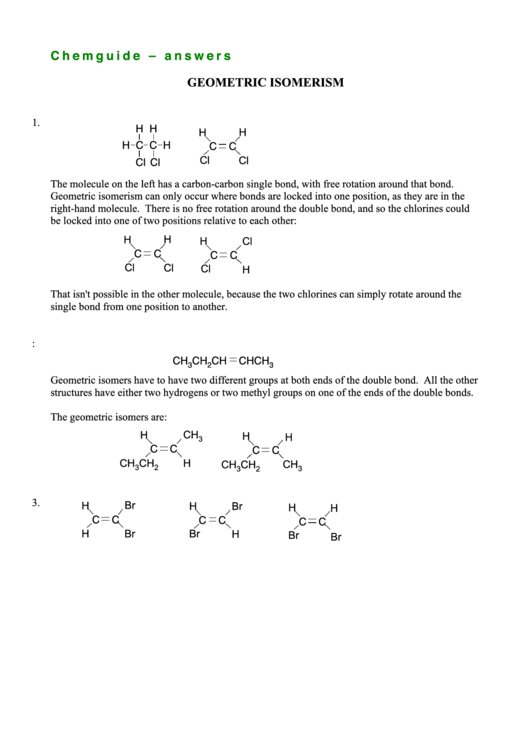
1.
H H
H
H
H C C H
C
C
Cl
Cl
Cl
Cl
The molecule on the left has a carbon-carbon single bond, with free rotation around that bond.
Geometric isomerism can only occur where bonds are locked into one position, as they are in the
right-hand molecule. There is no free rotation around the double bond, and so the chlorines could
be locked into one of two positions relative to each other:
H
H
H
Cl
C
C
C
C
Cl
Cl
Cl
H
That isn't possible in the other molecule, because the two chlorines can simply rotate around the
single bond from one position to another.
2. This is the only one which can have geometric isomers:
CH
CH
CH
CHCH
3
2
3
Geometric isomers have to have two different groups at both ends of the double bond. All the other
structures have either two hydrogens or two methyl groups on one of the ends of the double bonds.
The geometric isomers are:
H
CH
H
H
3
C
C
C
C
CH
CH
H
CH
CH
CH
3
2
3
2
3
3.
H
Br
H
Br
H
H
C
C
C
C
C
C
H
Br
Br
H
Br
Br
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2