Reading The Solubility Chart Worksheet Template
ADVERTISEMENT
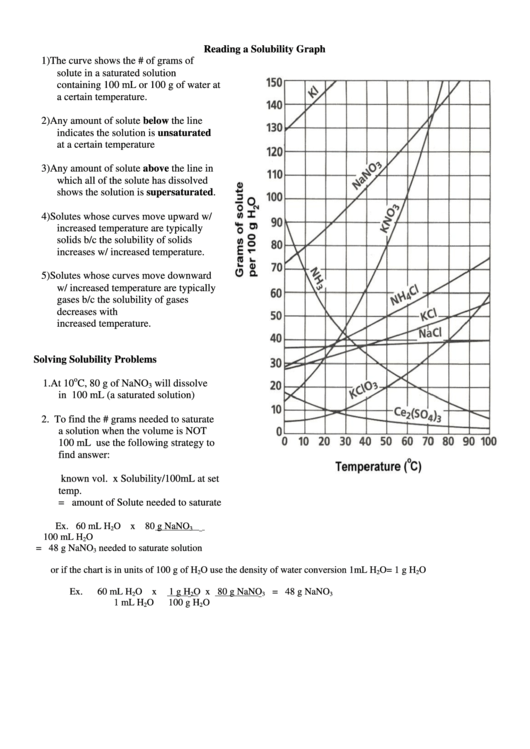
Reading a Solubility Graph
1) The curve shows the # of grams of
solute in a saturated solution
containing 100 mL or 100 g of water at
a certain temperature.
2) Any amount of solute below the line
indicates the solution is unsaturated
at a certain temperature
3) Any amount of solute above the line in
which all of the solute has dissolved
shows the solution is supersaturated.
4) Solutes whose curves move upward w/
increased temperature are typically
solids b/c the solubility of solids
increases w/ increased temperature.
5) Solutes whose curves move downward
w/ increased temperature are typically
gases b/c the solubility of gases
decreases with
increased temperature.
Solving Solubility Problems
o
1. At 10
C, 80 g of NaNO
will dissolve
3
in 100 mL (a saturated solution)
2. To find the # grams needed to saturate
a solution when the volume is NOT
100 mL use the following strategy to
find answer:
known vol. x Solubility/100mL at set
temp.
= amount of Solute needed to saturate
Ex. 60 mL H
O x 80 g NaNO
2
3
100 mL H
O
2
= 48 g NaNO
needed to saturate solution
3
or if the chart is in units of 100 g of H
O use the density of water conversion 1mL H
O= 1 g H
O
2
2
2
Ex.
60 mL H
O x
1 g H
O x 80 g NaNO
= 48 g NaNO
2
2
3
3
1 mL H
O
100 g H
O
2
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








