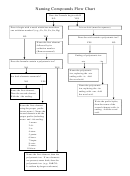
Naming Chemical Compounds Page 4
ADVERTISEMENT
there is only one of that atom. The mono prefix in carbon monoxide is used
because it has been in common use since the early days of chemistry.
The
compound NO is simply called nitrogen oxide.
CO
is then carbon dioxide, N
S
is dinitrogen trisulfide, PF
is phosphorous
2
2
3
5
pentasulfide, and so on. Elements are also named in this way, since they can exist
in multiple forms, called allotropes. For example, oxygen is found in nature as
both O
and O
. While these two gases are commonly called oxygen and ozone,
2
3
respectively, their official names are dioxygen and trioxygen.
Sample Problems
1.
Write the name for Cu(NO
)
.
2
2
Since the nitrite ion has a charge of 1-, the total negative charge is 2-. Thus,
copper has a 2+ charge and the name is copper(II) nitrite.
2.
Write the formula for aluminum sulfite.
The two ions in this compound are Al
3+
and SO
2-
. To balance the charges,
3
two aluminum ions and three sulfite ions will be required. Thus, the
formula will be Al
(SO
)
. Remember that parentheses must be use if there
2
3
2
is more than one of the polyatomic ion.
3.
Write the formula for tetraphosphorus hexoxide.
Since the prefix tetra means 4 and hexa means 6, the formula is P
O
.
4
6
4.
Write the formula for calcium dihydrogenarsenite.
-
2+
The two ions in this compound are Ca
and H
AsO
.
To balance the
2
3
charges, two anions will be needed to balance the cation. Thus the formula
will be Ca(H
AsO
)
.
2
3
2
5.
Write the name for Au(IO)
.
3
The hypoiodite ion has a 1- charge, so gold will have a 3+ charge to balance
the three anions. Thus the name will be gold(III) hypoiodite.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4