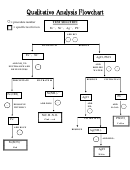
Qualitative Analysis Worksheet Page 2
ADVERTISEMENT
Part B
Question 3a: Based upon your observations from Part A, which ion(s) precipitate when HCl is
added?
Question 3b: Which ion(s) remain in the supernatant?
Question 4a: Based upon the solubility rules in the introduction, which ion(s) remaining in the
supernatant from step 4 will precipitate in basic solution?
Question 4b: Which ion(s) remain in the supernatant from step 5?
Question 5a: Based upon your observations in Part A and logic, which ion(s) remaining in the
supernatant from step 6 will precipitate when H
SO
is added?
2
4
Question 5b: Which ion(s) remain in the supernatant from step 7?
2
2010-2013 Advanced Instructional Systems, Inc. and North Carolina State University
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4