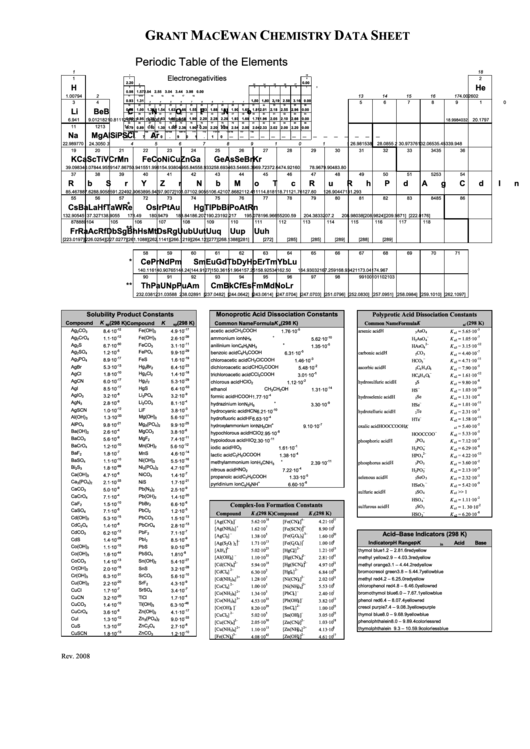
Grant Macewan Chemistry Data Sheet Periodic Table Of The Elements
ADVERTISEMENT
G
M
E
C
D
S
RANT
AC
WAN
HEMISTRY
ATA
HEET
Periodic Table of the Elements
1
18
1
Electronegativities
18
1
2
1
2
2.20
0.00
H
2
13
14
15
16
17
He
3
4
5
6
7
8
9
10
0.98 1.57
2.04 2.55 3.04 3.44 3.98 0.00
1.00794
2
13
14
15
16
17
4.002602
11
12
13
14
15
16
17
18
0.93 1.31
1.50 1.80 2.19 2.58 3.16 0.00
3
4
5
6
7
8
9
10
3
4
5
6
7
8
9
10
11
12
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
Li
Be
B
C
N
O
F
Ne
0.82 1.00 1.36 1.54 1.63 1.66 1.55 1.83 1.88 1.91 1.90 1.65 1.81 2.01 2.18 2.55 2.96 0.00
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
0.82 0.95 1.22 1.33 1.60 2.16 1.90 2.20 2.28 2.20 1.93 1.69 1.78 1.96 2.05 2.10 2.66 0.00
6.941
9.012182
10.811
12.0107
14.0067
15.9994
18.9984032
20.1797
55
56
57
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
11
12
13
14
15
16
17
18
0.79 0.89 1.10 1.30 1.50 2.36 1.90 2.20 2.20 2.28 2.54 2.00 2.04 2.33 2.02 2.00 2.20 0.00
Na
Mg
87
88
89
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
Al
Si
P
S
Cl
Ar
0.70 0.90 1.10
—
—
—
—
—
—
—
—
—
—
—
—
—
—
—
22.989770
24.3050
3
4
5
6
7
8
9
10
11
12
26.981538
28.0855
30.973761
32.065
35.453
39.948
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
39.0983
40.078
44.95591
47.867
50.9415
51.9961
54.938049
55.845
58.9332
58.6934
63.546
65.39
69.723
72.64
74.92160
78.96
79.904
83.80
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
I
Xe
85.4678
87.62
88.90585
91.224
92.90638
95.94
[97.9072]
101.07
102.9055
106.42
107.8682
112.411
114.818
118.71
121.76
127.60
126.90447
131.293
55
56
57
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
*
Cs
Ba
La
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
At
Rn
132.90545
137.327
138.9055
178.49
180.9479
183.84
186.207
190.23
192.217
195.078
196.96655
200.59
204.3833
207.2
208.98038 [208.9824] [209.9871] [222.0176]
87
88
89
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
**
Fr
Ra
Ac
Rf
Db
Sg
Bh
Hs
Mt
Ds
Rg
Uub
Uut
Uuq
Uup
Uuh
[223.0197] [226.0254] [227.0277] [261.1088] [262.1141] [266.1219]
[264.12]
[277]
[268.1388]
[281]
[272]
[285]
[285]
[289]
[288]
[289]
58
59
60
61
62
63
64
65
66
67
68
69
70
71
*
Ce
Pr
Nd
Pm
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
140.116
140.90765
144.24
[144.9127]
150.36
151.964
157.25
158.92534
162.50
164.93032
167.259
168.93421
173.04
174.967
90
91
92
93
94
95
96
97
98
99
100
101
102
103
**
Th
Pa
U
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
Lr
232.0381 231.03588 238.02891 [237.0482] [244.0642] [243.0614] [247.0704] [247.0703] [251.0796] [252.0830] [257.0951] [258.0984] [259.1010] [262.1097]
Solubility Product Constants
Monoprotic Acid Dissociation Constants
Polyprotic Acid Dissociation Constants
K
(298 K)
K
(298 K)
K
(298 K)
K
Compound
Compound
Common Name
Formula
Common Name
Formula
(298 K)
sp
sp
a
ai
-12
-17
-3
Ag
CO
Fe(OH)
CH
COOH
-5
H
AsO
8.4·10
4.9·10
acetic acid
arsenic acid
K
= 5.65·10
1.76·10
2
3
2
3
3
4
a1
Ag
CrO
-12
Fe(OH)
-39
+
-10
–
-7
ammonium ion
1.1·10
2.6·10
NH
5.62·10
H
AsO
K
= 1.05·10
2
4
3
4
2
4
a2
Ag
S
-50
FeCO
-11
+
-5
2–
-12
6.7·10
3.1·10
anilinium ion
C
H
NH
HAsO
K
= 3.15·10
1.35·10
2
3
6
5
3
4
a3
-5
-29
Ag
SO
FePO
-5
-7
1.2·10
9.9·10
C
H
COOH
H
CO
benzoic acid
carbonic acid
K
= 4.40·10
6.31·10
2
4
4
6
5
2
3
a1
Ag
PO
-17
-19
FeS
CH
ClCOOH
-3
–
-11
8.9·10
1.6·10
chloroacetic acid
1.46·10
HCO
K
= 4.71·10
3
4
2
3
a2
-13
Hg
Br
-23
AgBr
-5
5.3·10
6.4·10
CHCl
COOH
-2
H
C
H
O
dichloroacetic acid
ascorbic acid
K
= 7.90·10
5.48·10
2
2
2
2
6
6
6
a1
AgCl
-10
Hg
Cl
-18
1.8·10
1.4·10
-1
–
-12
trichloroacetic acid
CCl
COOH
3.01·10
HC
H
O
K
= 1.61·10
2
2
3
6
6
6
a2
-17
Hg
I
-29
AgCN
6.0·10
5.3·10
HClO
-2
-8
chlorous acid
hydrosulfuric acid
H
S
K
= 9.80·10
1.12·10
2
2
2
2
a1
-17
-53
AgI
HgS
8.5·10
6.4·10
-14
-19
ethanol
CH
CH
OH
–
1.31·10
K
= 1.03·10
HS
3
2
a2
AgIO
-8
Li
PO
-9
3.2·10
3.2·10
-4
-4
formic acid
HCOOH
hydroselenic acid
H
Se
3
3
4
1.77·10
K
= 1.31·10
2
a1
AgN
-9
Li
CO
-4
2.8·10
8.1·10
+
-9
-11
hydrazinium ion
–
N
H
3.30·10
K
= 1.01·10
3
2
3
HSe
2
5
a2
-12
-3
AgSCN
LiF
1.0·10
3.8·10
-10
-3
hydrocyanic acid
HCN
hydrotelluric acid
H
Te
6.21·10
K
= 2.31·10
2
a1
Al(OH)
-33
Mg(OH)
-11
1.3·10
5.6·10
-4
-11
–
hydrofluoric acid
HF
K
= 1.58·10
3
2
6.63·10
HTe
a2
AlPO
-21
Mg
(PO
)
-25
9.8·10
9.9·10
+
-7
-2
hydroxylammonium ion
NH
OH
oxalic acid
HOOCCOOH
4
3
4
2
9.10·10
K
= 5.40·10
3
a1
Ba(OH)
-4
MgCO
-6
2.6·10
3.8·10
-8
–
-5
2
3
hypochlorous acid
HClO
2.95·10
HOOCCOO
K
= 5.33·10
a2
BaCO
-9
MgF
-11
5.6·10
7.4·10
-11
-3
hypoiodous acid
HIO
H
PO
3
2
2.30·10
phosphoric acid
K
= 7.12·10
3
4
a1
-10
-12
BaCrO
Mn(OH)
1.2·10
5.6·10
HIO
-1
–
-8
4
2
iodic acid
1.61·10
H
PO
K
= 6.29·10
3
2
4
a2
BaF
-7
-14
MnS
1.8·10
4.6·10
-4
2–
-13
2
lactic acid
C
H
OCOOH
1.38·10
HPO
K
= 4.22·10
2
5
4
a3
BaSO
-10
Ni(OH)
-16
1.1·10
5.5·10
+
-11
-2
4
2
methylammonium ion
H
CNH
H
PO
2.39·10
phosphorus acid
K
= 3.60·10
3
3
3
3
a1
-99
-32
Bi
S
Ni
(PO
)
1.8·10
4.7·10
-4
2
3
3
4
2
nitrous acid
HNO
–
-7
7.22·10
H
PO
K
= 2.13·10
2
2
3
a2
Ca(OH)
-6
NiCO
-7
4.7·10
1.4·10
C
H
COOH
-5
-3
2
3
propanoic acid
H
SeO
1.33·10
selenous acid
K
= 2.32·10
2
5
2
3
a1
Ca
(PO
)
-33
-21
NiS
2.1·10
1.7·10
+
3
4
2
-6
–
-9
pyridinium ion
C
H
NH
6.60·10
HSeO
K
= 5.42·10
5
5
3
a2
CaCO
-9
Pb(N
)
-9
5.0·10
2.5·10
3
3
2
H
SO
K
>> 1
sulfuric acid
2
4
a1
CaCrO
-4
Pb(OH)
-20
7.1·10
1.4·10
4
2
–
-2
HSO
K
= 1.11·10
4
a2
-10
-6
CaF
PbBr
1.5·10
6.6·10
Complex-Ion Formation Constants
2
2
-2
sulfurous acid
H
SO
K
= 1. 30·10
2
3
a1
CaSO
-5
PbCl
-5
7.1·10
1.2·10
4
2
K
(298 K)
K
(298 K)
–
-8
Compound
Compound
HSO
K
= 6.20·10
f
f
3
a2
Cd(OH)
-15
PbCO
-13
5.3·10
1.5·10
2
3
–
18
4–
37
[Ag(CN)
]
5.62·10
[Fe(CN)
]
4.21·10
2
6
-8
-13
CdC
O
PbCrO
1.4·10
2.8·10
2
4
4
+
7
2+
2
[Ag(NH
)
]
1.62·10
[Fe(SCN)]
8.90·10
3
2
CdCO
-12
PbF
-7
6.2·10
7.1·10
Acid–Base Indicators (298 K)
3
2
–
3–
5
20
[AgCl
]
[Fe(C
O
)
]
1.38·10
1.60·10
2
2
4
3
-29
PbI
-9
CdS
1.4·10
8.5·10
2
3–
13
+
8
Indicator
pH Range
pK
Acid
Base
[Ag(S
O
)
]
1.71·10
[Fe(C
O
)]
1.00·10
2
3
2
2
4
in
-15
-29
Co(OH)
PbS
1.1·10
9.0·10
2
3–
2–
23
15
[AlF
]
[HgCl
]
5.02·10
1.21·10
thymol blue
1.2 – 2.8
1.6
red
yellow
6
4
Co(OH)
-44
PbSO
-8
1.6·10
1.810
3
4
–
2–
33
41
[Al(OH)
]
[Hg(CN)
]
1.10·10
2.81·10
methyl yellow
2.9 – 4.0
3.3
red
yellow
4
4
-13
-27
CoCO
Sn(OH)
1.4·10
5.4·10
3
2
2–
18
2–
21
[Cd(CN)
]
5.94·10
[Hg(SCN)
]
4.97·10
methyl orange
3.1 – 4.4
4.2
red
yellow
4
4
Cr(OH)
-16
-28
SnS
2.0·10
3.2·10
2
2–
2–
2
29
[CdCl
]
[HgI
]
bromocresol green
3.8 – 5.4
4.7
yellow
blue
6.30·10
6.84·10
4
4
Cr(OH)
-31
SrCO
-10
6.3·10
5.6·10
3
3
2+
2–
7
31
[Cd(NH
)
]
[Ni(CN)
]
methyl red
4.2 – 6.2
5.0
red
yellow
1.28·10
2.02·10
3
4
4
-20
-9
Cu(OH)
SrF
2.2·10
4.3·10
2
2
2–
3
2+
8
chlorophenol red
4.8 – 6.4
6.0
yellow
red
[CoCl
]
1.00·10
[Ni(NH
)
]
5.53·10
4
3
6
-7
SrSO
-7
CuCl
1.7·10
3.4·10
4
2+
–
5
1
[Co(NH
)
]
[PbCl
]
bromothymol blue
6.0 – 7.6
7.1
yellow
blue
1.34·10
2.40·10
3
6
3
-20
-4
CuCN
TlCl
3.2·10
1.7·10
3+
33
–
13
[Co(NH
)
]
[Pb(OH)
]
phenol red
6.4 – 8.0
7.4
yellow
red
4.53·10
3.82·10
3
6
3
-10
-46
CuCO
Tl(OH)
1.4·10
6.3·10
3
3
–
29
2–
25
cresol purple
7.4 – 9.0
8.3
yellow
purple
[Cr(OH)
]
8.20·10
[SnCl
]
1.00·10
4
6
CuCrO
-6
Zn(OH)
-17
3.6·10
4.1·10
4
2
2–
–
5
25
thymol blue
8.0 – 9.6
8.9
yellow
blue
[CuCl
]
[Sn(OH)
]
5.02·10
3.05·10
3
3
-12
-33
CuI
Zn
(PO
)
1.3·10
9.0·10
3
4
2
2–
30
2–
18
phenolphthalein
8.0 – 9.8
9.4
colorless
red
[Cu(CN)
]
[Zn(CN)
]
2.05·10
1.03·10
4
4
-37
ZnC
O
-8
CuS
1.3·10
2.7·10
2
4
2+
2+
thymolphthalein
9.3 – 10.5
9.9
colorless
blue
13
8
[Cu(NH
)
]
1.10·10
[Zn(NH
)
]
4.13·10
3
4
3
4
-13
ZnCO
-10
CuSCN
1.8·10
1.2·10
3
3–
2–
42
17
[Fe(CN)
]
[Zn(OH)
]
4.08·10
4.61·10
6
4
Rev. 2008
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








