Certificate Of Service
ADVERTISEMENT
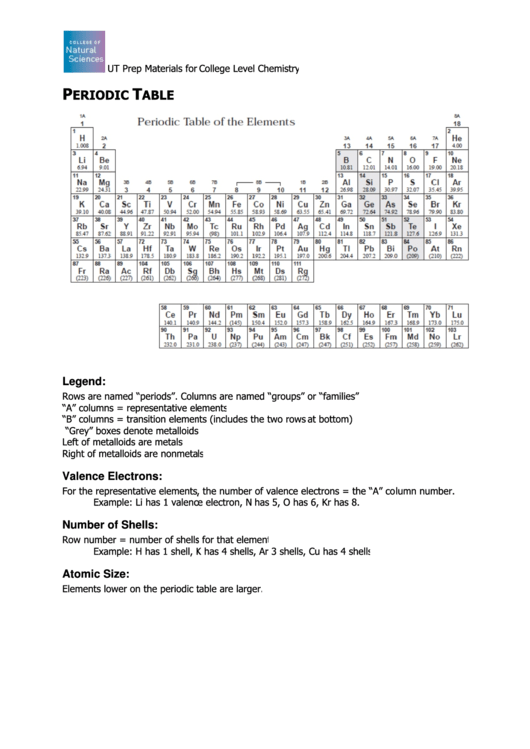
UT Prep Materials for College Level Chemistry
UT Prep Materials for College Level Chemistry
P
T
ERIODIC
ABLE
Legend:
Rows are named “periods”. Columns are named “groups” or “families”.
Rows are named “periods”. Columns are named “groups” or “families”.
“A” columns = representative elements
“A” columns = representative elements
“B” columns = transition elements (includes the two rows
“B” columns = transition elements (includes the two rows at bottom)
“Grey” boxes denote metalloids
Left of metalloids are metals
Right of metalloids are nonmetals
Right of metalloids are nonmetals
Valence Electrons:
For the representative elements, the number of valence electrons = the “A” column number.
For the representative elements, the number of valence electrons = the “A” column number.
For the representative elements, the number of valence electrons = the “A” column number.
Example: Li has 1 valence electron, N
Example: Li has 1 valence electron, N has 5, O has 6, Kr has 8.
Number of Shells:
Row number = number of shells for that element
Row number = number of shells for that element
Example: H has 1 shell, K has 4 shells, Ar 3 shells, Cu has 4 shells
Example: H has 1 shell, K has 4 shells, Ar 3 shells, Cu has 4 shells
Atomic Size:
Elements lower on the periodic table are larger.
Elements lower on the periodic table are larger.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








