Ionic Compound Practice 1
ADVERTISEMENT
Ionic Compound Practice 1
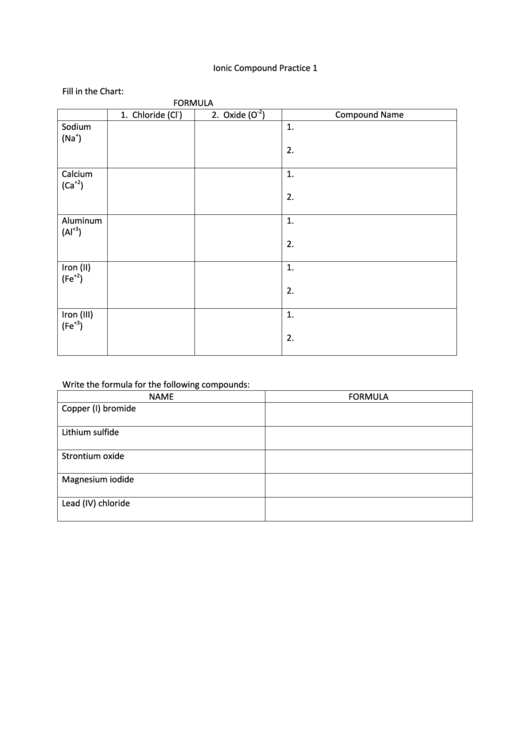
Fill in the Chart:
FORMULA
-
-2
1. Chloride (Cl
)
2. Oxide (O
)
Compound Name
Sodium
1.
+
(Na
)
2.
Calcium
1.
+2
(Ca
)
2.
Aluminum
1.
+3
(Al
)
2.
Iron (II)
1.
+2
(Fe
)
2.
Iron (III)
1.
+3
(Fe
)
2.
Write the formula for the following compounds:
NAME
FORMULA
Copper (I) bromide
Lithium sulfide
Strontium oxide
Magnesium iodide
Lead (IV) chloride
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








