Solubility Rules For Ionic Compounds At 25c
ADVERTISEMENT
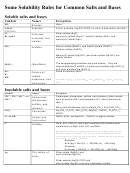
Solubility Rules for Ionic Compounds at 25°C
Soluble Compounds
Exceptions
Almost all salts of sodium, potassium
None
the other alkali metals,
and ammonium
All salts of chloride, bromide, and iodide
Halides salts of silver, mercury (I),
and lead (II)
Compounds containing fluoride
Fluorides of magnesium, calcium,
strontium, barium, and lead (II).
Salts of nitrate, chlorate, perchlorate,
None
bicarbonate, and acetate
Salts of sulfate
Sulfates of silver, strontium, barium,
lead (II), and calcium
Inorganic acids
Insoluble Compounds
Exceptions
All salts of carbonate, phosphate,
Salts of ammonium and the alkali
oxalate, and chromate
metal cations
Compounds containing sulfide
Salts of ammonium, calcium,
strontium, barium, and the alkali
metal cations.
Metal hydroxides and oxides
Hydroxides or oxides of barium
and the alkali metal cations.
(Note: NH
OH does not exist in
4
ionic form: it is actually NH
in
3
water.)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1