Worksheet - Stoichiometry (Mole-Mole; Mole-Mass) Page 2
ADVERTISEMENT
3. The first step in the industrial manufacture of nitric acid involves the catalytic oxidation of
ammonia according to the following BALANCED equation.
4NH
(g) + 5O
(g)
4NO (g) + 6H
O (g)
3
2
2
a. How many moles of NO are formed if 824 g of NH
react?
3
b. How many grams of water are formed if 2.5 moles of ammonia are oxidized?
c. How many moles of oxygen are needed to react with 4.6 moles of ammonia?
4. Mercury (II) oxide decomposes into mercury and oxygen gas according to the following
UNBALANCED equation.
_____HgO
_____ Hg + _____O
2
a. How many moles of mercury (II) oxide are needed to produce 125 g of oxygen?
b. How many moles of mercury are produced if 24.5 moles of mercury (II) oxide decompose?
c. How many grams of oxygen will be produced if 2.3 moles of mercury are produced?
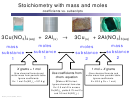
5. Use the following equation to answer the questions below.
_____Cu + ____O
____CuO
2
a. If 101 grams of copper are used, how many moles of copper (II) oxide will be formed?
b. If 5.25 moles of copper are used, how many moles of oxygen must also be used?
c. If 78.2 grams of oxygen react with copper, how many moles of copper (II) oxide will be
produced?
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3