Writing And Balancing Equations Page 4
ADVERTISEMENT
Workshop 4, page 4
Single Replacement Reactions: Activity Series
Li > K > Ba > Sr > Ca > Na > Mg > Al > Mn > Zn > Fe > Cd > Co > Ni > Sn > Pb
The above elements all replace hydrogen from acids.
The most active can replace H from water (Li – Na) . Mg can slowly react with
hot water.
Al – Pb react with acids but not with water.
H > Cu > Ag > Hg > Au
(Cu, Hg, Ag, do not replace H from acids)
Predict whether the following will react, and what the products will be if they do. If no
reaction is predicted, write NR. Balance the equations
→
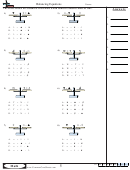
9.
H
+ CuO
2
→
10.
Mg + MnCl
2
→
11.
Mg + AgNO
3
→
12.
K + Na
SiO
2
3
→
13.
Cu +
Fe
O
2
3
Decomposition Reactions
→
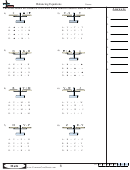
14.
Mg (OH)
2
→
15.
H
CO
2
3
→
16.
LiClO
4
Combination reactions
→
17.
P
O
+
H
O
2
3
2
→
18.
SO
+
H
O
2
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4