Writing And Balancing Chemical Reactions, Stoichiometry, Limiting Reactants Worksheet With Answers - Chem110
ADVERTISEMENT
CHEM110 Week 5 Notes (Stoichiometry)
Page 1 of 8
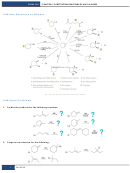
Writing and Balancing Chemical Reactions
A chemical reaction shows the reactants needed, products formed, and states symbols for
all the ingredients. Chemicals must be written correctly based on ionic charge and
information given in the problem. Only after that is done correctly can a chemical
reaction be balanced. A balanced reaction has the same atom count on both sides, and
this is required by the law of conservation of matter.
Ca(s) + I
(s) →
CaI
(s)
2
2
Reactants
Products
To balance a reaction, use coefficients (full-sized numbers before a chemical) to get the
atom count the same on the left (reactants) and right (products) side of the reaction arrow.
Coefficients multiply everything that comes after them in that particular chemical. In this
case, the reaction is balanced because the atom count is the same on both sides.
Ca(s) + I
(s) →
CaI
(s)
2
2
__1__ Ca __1__
__2__ I
__2__
Atom count on left side
Atom count on right side
Because the atom count is already the same on both sides, no coefficients are needed to
balance the reaction. The coefficients are all one (1). The reaction says that 1 atom of
solid calcium reacts with 1 molecule of solid iodine to give 1 formula unit of solid
calcium iodide. Equivalently, the reaction tells us that 1 mole of solid calcium reacts with
1 mole of solid iodine to give 1 mole of solid calcium iodide.
Many reactions require coefficients (or stoichiometric coefficients) to get the atom count
the same on both sides. Balance this reaction:
Mg(s) + N
(s)
→
Mg
N
(s)
2
3
2
__1__ Mg __3__
__2__ N
__2__
The magnesium count is not balanced. Add a 3 coefficient before Mg on the left side to
balance the reaction.
3 Mg(s) + N
(s)
→
Mg
N
(s)
2
3
2
__3__ Mg __3__
__2__ N
__2__
Balance the following reaction (recall that O
is diatomic):
2
HgO(s)
→
Hg(l) + O
(g)
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8