Naming Compounds That Contain Polyatomic Ions Worksheet
ADVERTISEMENT
Page 1 of 4
Focus Questions
Sections 4.1–4.3
1. Why is a system for naming compounds necessary?
2. How can you tell if a substance is a binary compound?
3. How are the anions in all types of binary compounds similar?
4. How are the cations different in each type of binary compound?
5. Use Figure 4.1 to name the following compounds:
a. KBr
d. CuBr
b. SnF
e. MgI
2
2
c. CO
f. PCl
3
Naming Compounds That Contain
Polyatomic Ions
Objective:
To learn the names of common polyatomic ions and how to use
them in naming compounds.
A
type of ionic compound that we have not yet considered is exemplified
by ammonium nitrate, NH
NO
, which contains the
polyatomic ions
4
3
CHEMISTRY
NH
and NO
. As their name suggests, polyatomic ions are charged enti-
4
3
ties composed of several atoms bound together. Polyatomic ions are assigned
Ionic compounds containing
polyatomic ions are not binary
special names that you must memorize to name the compounds containing
compounds, because they con-
them. The most important polyatomic ions and their names are listed in
tain more than two elements.
Table 4.4.
Note in Table 4.4 that several series of polyatomic anions exist that con-
tain an atom of a given element and different numbers of oxygen atoms.
These anions are called
oxyanions.
When there are two members in such a
series, the name of the one with the smaller number of oxygen atoms ends
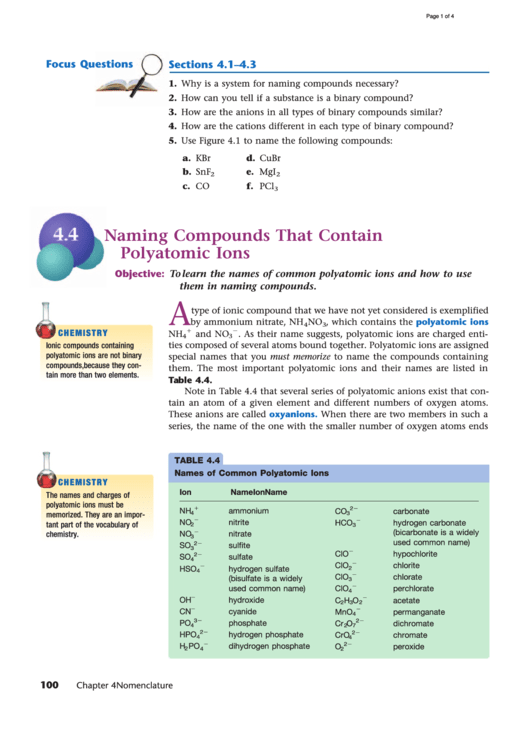
TABLE 4.4
Names of Common Polyatomic Ions
CHEMISTRY
Ion
Name
Ion
Name
The names and charges of
polyatomic ions must be
2
NH
ammonium
CO
carbonate
4
3
memorized. They are an impor-
NO
nitrite
HCO
hydrogen carbonate
tant part of the vocabulary of
2
3
(bicarbonate is a widely
NO
nitrate
chemistry.
3
used common name)
2
SO
sulfite
3
ClO
hypochlorite
2
SO
sulfate
4
ClO
chlorite
HSO
hydrogen sulfate
2
4
ClO
chlorate
(bisulfate is a widely
3
used common name)
ClO
perchlorate
4
OH
hydroxide
C
H
O
acetate
2
3
2
CN
cyanide
MnO
permanganate
4
3
2
PO
phosphate
Cr
O
dichromate
4
2
7
2
2
HPO
hydrogen phosphate
CrO
chromate
4
4
2
H
PO
dihydrogen phosphate
O
peroxide
2
4
2
100
Chapter 4 Nomenclature
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4








