Gas Transport In The Blood
ADVERTISEMENT
B. 7 Gas transport in the blood
a. Describe the carriage of oxygen in blood.
Oxygen is carried either bound to haemoglobin or dissolved in solution. The
solubility of oxygen in blood is 0.003 ml/100 ml/mmHg so normal arterial blood contains
about 0.3 ml/100 ml. The large proportion of oxygen in the blood is bound to haemoglobin, a
protein tetramer with an iron-porphyrin ring attached to each chain. Oxygen coordinates
with each Fe atom, inducing a conformational change which promotes the binding of oxygen
to the other Fe atoms. The total oxygen binding capacity of haemoglobin in blood (at normal
pH, temperature and P
) is 1.39ml/g, giving a total oxygen carrying capacity of blood with
CO
2
an Hb of 150 g/l of 20.8 ml/100 ml. Normal arterial blood has a P
of 100 mmHg and is
O
2
97.5% saturated; venous blood has a P
of 40 mmHg and is 75% saturated.
O
2
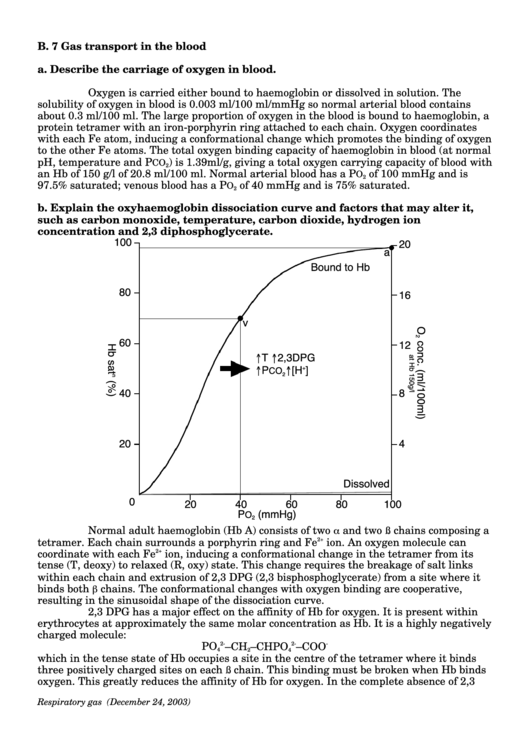
b. Explain the oxyhaemoglobin dissociation curve and factors that may alter it,
such as carbon monoxide, temperature, carbon dioxide, hydrogen ion
concentration and 2,3 diphosphoglycerate.
100
20
a
Bound to Hb
80
16
v
60
12
↑T ↑2,3DPG
+
]
↑P
↑[H
CO
2
40
8
20
4
Dissolved
0
20
40
60
80
100
P
(mmHg)
O
2
Normal adult haemoglobin (Hb A) consists of two
and two
chains composing a
ß
α
tetramer. Each chain surrounds a porphyrin ring and Fe
2+
ion. An oxygen molecule can
coordinate with each Fe
2+
ion, inducing a conformational change in the tetramer from its
tense (T, deoxy) to relaxed (R, oxy) state. This change requires the breakage of salt links
within each chain and extrusion of 2,3 DPG (2,3 bisphosphoglycerate) from a site where it
binds both
chains. The conformational changes with oxygen binding are cooperative,
β
resulting in the sinusoidal shape of the dissociation curve.
2,3 DPG has a major effect on the affinity of Hb for oxygen. It is present within
erythrocytes at approximately the same molar concentration as Hb. It is a highly negatively
charged molecule:
PO
2-
–CH
–CHPO
2-
–COO
-
4
2
4
which in the tense state of Hb occupies a site in the centre of the tetramer where it binds
three positively charged sites on each
chain. This binding must be broken when Hb binds
ß
oxygen. This greatly reduces the affinity of Hb for oxygen. In the complete absence of 2,3
Respiratory gas transport
1.B.7.1
James Mitchell (December 24, 2003)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3








