Phase Change Diagram Science Worksheets
ADVERTISEMENT
Name________________________________
Date__________
Period_____
Phase Change Diagram
Heat plays an important role in phase changes. Heat is energy that causes particles of matter
to move faster and farther apart. As particles move faster, they leave one phase and enter another.
Phase changes produce changes in only the physical properties of matter. They do not produce
changes in the chemical properties. A substance is still the same kind of matter regardless of its
phase.
Examples of phase changes include melting, freezing, condensation, evaporation, and
sublimation. Melting occurs when a solid changes to a liquid. Freezing occurs when a liquid
becomes a solid. Condensation involves a gas becoming a liquid. Evaporation involves a liquid
becoming a gas and sublimation is the change of a solid directly to a gas. Phase changes require
either the addition of heat energy (melting, evaporation, and sublimation) or subtraction of heat
energy (condensation and freezing).
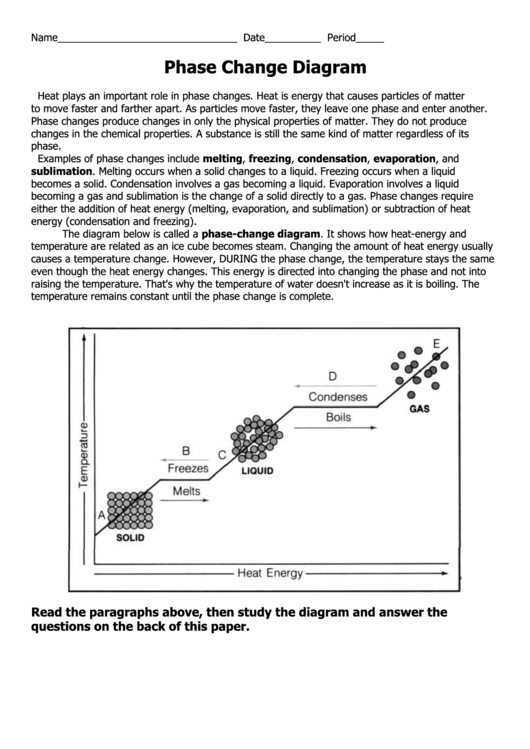
The diagram below is called a phase-change diagram. It shows how heat-energy and
temperature are related as an ice cube becomes steam. Changing the amount of heat energy usually
causes a temperature change. However, DURING the phase change, the temperature stays the same
even though the heat energy changes. This energy is directed into changing the phase and not into
raising the temperature. That's why the temperature of water doesn't increase as it is boiling. The
temperature remains constant until the phase change is complete.
Read the paragraphs above, then study the diagram and answer the
questions on the back of this paper.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2