Phase Changes Worksheets
ADVERTISEMENT
Name ___________________________
Class ___________________
Date _____________
Chapter 3
States of Matter
Section 3.3 Phase Changes
(pages 84–91)
This section explains what happens when a substance changes from one state
of matter to another and describes six phase changes.
Reading Strategy
(page 84)
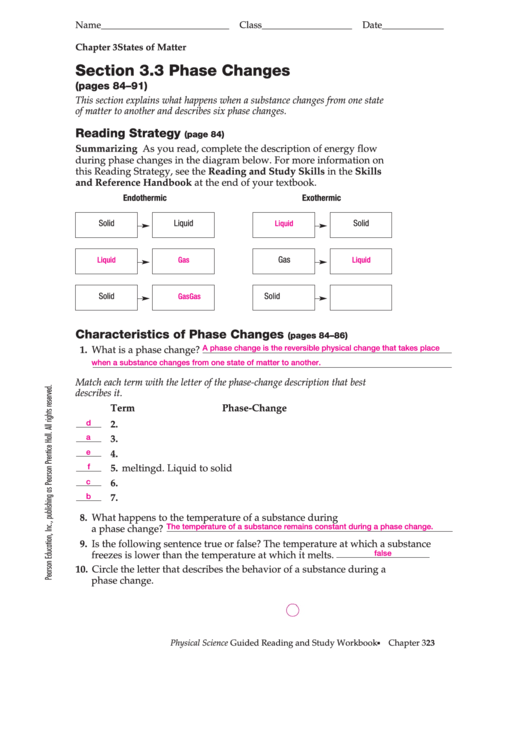
Summarizing As you read, complete the description of energy flow
during phase changes in the diagram below. For more information on
this Reading Strategy, see the Reading and Study Skills in the Skills
and Reference Handbook at the end of your textbook.
Endothermic
Exothermic
Solid
Liquid
Solid
Liquid
Gas
Liquid
Gas
Liquid
Solid
Solid
Gas
Gas
Characteristics of Phase Changes
(pages 84–86)
A phase change is the reversible physical change that takes place
1. What is a phase change?
when a substance changes from one state of matter to another.
Match each term with the letter of the phase-change description that best
describes it.
Term
Phase-Change
d
2. freezing
a. Solid to gas
a
3. sublimation
b. Liquid to gas
e
4. condensation
c. Gas to solid
f
5. melting
d. Liquid to solid
c
6. deposition
e. Gas to liquid
b
7. vaporization
f. Solid to liquid
8. What happens to the temperature of a substance during
The temperature of a substance remains constant during a phase change.
a phase change?
9. Is the following sentence true or false? The temperature at which a substance
false
freezes is lower than the temperature at which it melts.
10. Circle the letter that describes the behavior of a substance during a
phase change.
a. neither absorbs nor releases energy
b. always absorbs energy
c. always releases energy
d. either absorbs or releases energy
Physical Science Guided Reading and Study Workbook
Chapter 3
23
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2