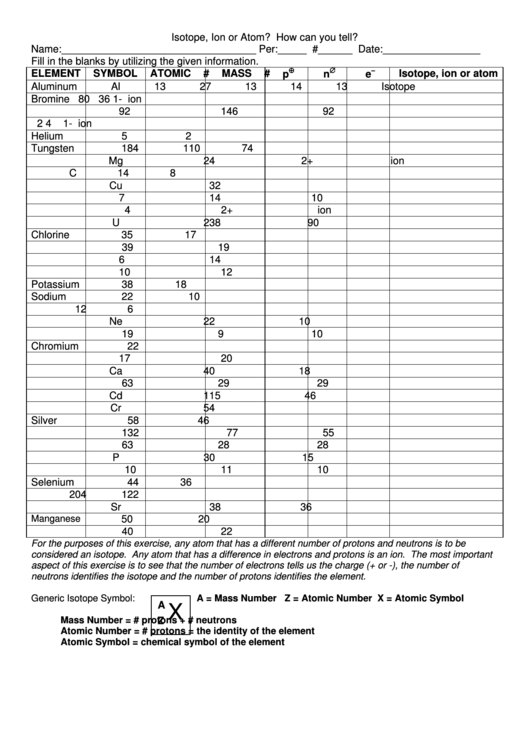
Isotope, Ion Or Atom
ADVERTISEMENT
Isotope, Ion or Atom? How can you tell?
Name:__________________________________ Per:_____ #______ Date:_________________
Fill in the blanks by utilizing the given information.
⊕
∅
−
ELEMENT
SYMBOL
ATOMIC #
MASS #
Isotope, ion or atom
p
n
e
Aluminum
Al
13
27
13
14
13
Isotope
Bromine
80
36
1- ion
92
146
92
2
4
1- ion
Helium
5
2
Tungsten
184
110
74
Mg
24
2+ ion
C
14
8
Cu
32
7
14
10
4
2+ ion
U
238
90
Chlorine
35
17
39
19
6
14
10
12
Potassium
38
18
Sodium
22
10
12
6
Ne
22
10
19
9
10
Chromium
22
17
20
Ca
40
18
63
29
29
Cd
115
46
Cr
54
Silver
58
46
132
77
55
63
28
28
P
30
15
10
11
10
Selenium
44
36
204
122
Sr
38
36
Manganese
50
20
40
22
For the purposes of this exercise, any atom that has a different number of protons and neutrons is to be
considered an isotope. Any atom that has a difference in electrons and protons is an ion. The most important
aspect of this exercise is to see that the number of electrons tells us the charge (+ or -), the number of
neutrons identifies the isotope and the number of protons identifies the element.
Generic Isotope Symbol:
A = Mass Number Z = Atomic Number X = Atomic Symbol
A
X
Z
Mass Number = # protons + # neutrons
Atomic Number = # protons = the identity of the element
Atomic Symbol = chemical symbol of the element
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








