Resonance Worksheet Page 4
ADVERTISEMENT
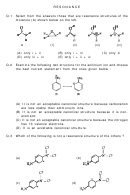
SOLUTIONS (RESONANCE)
Ans.1 In (i) & (ii), shifting of Hydrogen atom takes place which violates the
Resonance rule.
Ans.2 Nitrogen cannot expand its octet due to absence of vacant d-orbitals.
Ans.3 In (a), (b), (d) structures, net charge = +1
while in (c), net charge = 0
Net charge should be conserved in all the Resonating structure.
← →
← →
Ans.4
(a)
(b)
(c)
(a), (b), (c) structure can be drawn from each other while (d) can't
be drawn from rest of the three.
Ans.5 Net charge is not conserved in structure (c).
Ans.6 In (c), Nitrogen has 10 valence electrons.
Ans.7 In (iii) & (iv) cationic carbon is sp-hybridised positive charge is
unstable on these carbon atoms due to high electronegativity of sp-
hybrid orbitals.
Ans.8 In structure (B), carbon atom bearing —NH
group has 10 valence
2
electrons which violates the octet rule.
← →
Ans.9
Ans.10 The resonance structures of Hydrogen azide are as following :
← →
(A)
(B)
The bond order between N atom at location I is between 1 & 2.
whereas at location II the bond order is between 2 & 3.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4








