Solubility And Precipitation Worksheet
ADVERTISEMENT
Solubility and Precipitation
Introduction
Materials
Some combinations of ionic solutions produce insoluble products called
0.1 M solutions of the following
precipitates and some produce no visible reaction at all. In this investigation
chemicals (dispensed in
you will be performing several double replacement reactions by combining
labeled Beral pipets): AgNO
,
3
several pairs of dissolved ionic substances. Using a solubility chart, you
NaNO
, Na
PO
, K
CO
,
3
3
4
2
3
should be able to identify the precipitates that you observe. You also should
FeCl
, and CuSO
.
3
4
be able to recognize some trends in solubility of some ions.
well plate
goggles
Procedure
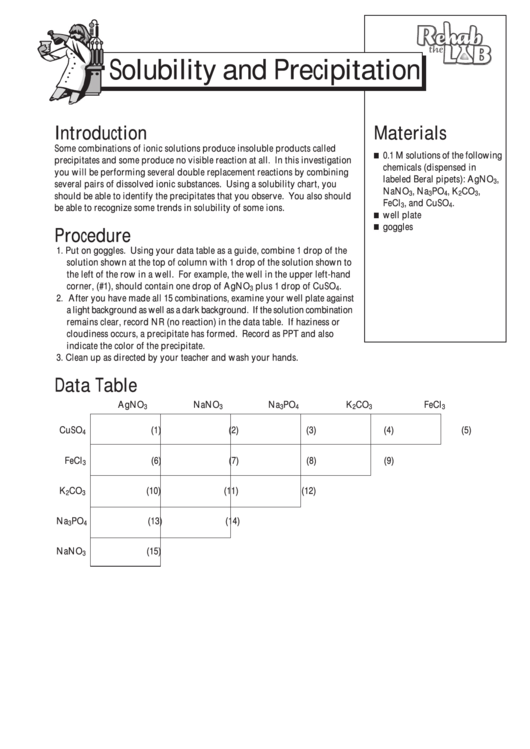
1. Put on goggles. Using your data table as a guide, combine 1 drop of the
solution shown at the top of column with 1 drop of the solution shown to
the left of the row in a well. For example, the well in the upper left-hand
corner, (#1), should contain one drop of AgNO
plus 1 drop of CuSO
.
3
4
2. After you have made all 15 combinations, examine your well plate against
a light background as well as a dark background. If the solution combination
remains clear, record NR (no reaction) in the data table. If haziness or
cloudiness occurs, a precipitate has formed. Record as PPT and also
indicate the color of the precipitate.
3. Clean up as directed by your teacher and wash your hands.
Data Table
AgNO
NaNO
Na
PO
K
CO
FeCl
3
3
3
4
2
3
3
CuSO
(1)
(2)
(3)
(4)
(5)
4
FeCl
(6)
(7)
(8)
(9)
3
K
CO
(10)
(11)
(12)
2
3
Na
PO
(13)
(14)
3
4
NaNO
(15)
3
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








