SPECIMEN REQUIREMENTS FOR ZIKA TESTING
Clinics able to process specimens may centrifuge blood and transfer serum to a separate, sterile labeled tube.
Note, requested sample volumes are for adults. Samples must be sent to the Los Angeles County Public
Health Laboratories as soon as possible and within 24 hours of collection.
Test
Specimen Type
Specimen Requirements
Storage and Transport
Conditions*
Zika IgM serology
Serum
(QTY 2)
Store at 4-8°C and immediately ship
5-7 mL plastic red top or gold
on cold pack
top serum separator tube**
CSF
2 mL collected in sterile
Store at 4-8°C and immediately ship
(if collected for
container
on cold pack
other purposes)
Zika real-time
Serum
(QTY 2)
Store at 4-8°C and immediately ship
RT-PCR
5-7 mL plastic red top or gold
on cold pack
top serum separator tube**
Note: Serum is the
CSF
2 mL collected in sterile
Store at 4-8°C and immediately ship
primary specimen type
(if collected for
container
on cold pack
for Zika PCR. Other
other purposes)
samples may be
requested depending on
Urine, random
10-20 mL collected in sterile
Store at 4-8°C and immediately ship
patient status and
container
on cold pack
history.
COLLECT THESE SPECIMEN TYPES ONLY UPON CONSULTATION
AND INSTRUCTION FROM PUBLIC HEALTH
If <7 days from onset of
Amniotic Fluid
5-10 mL collected in sterile
Store at 4-8°C and immediately ship
symptoms, submit both
container
on cold pack
urine and serum
Cord Blood
(QTY 2) 5-7 mL red top or
Store at 4-8°C and immediately ship
specimens; urine should
gold top serum separator
on cold pack
be collected within 14
tube**
days of symptom onset
Placenta
Intact if early gestation or
Store fresh sample at 4-8°C and
to improve sensitivity of
extensive sampling of full
immediately ship on cold pack. If
diagnosis, however
thickness pieces including
frozen, ship on dry ice. If fixed, ship
urine specimens
disk, membranes, umbilical
at room temperature.
collected within 30 days
cord, any pathologic lesions
will continue to be
***Fetal Tissue,
At minimum,
Store fresh sample at 4-8°C and
accepted.
fresh or frozen
1 cm
3
section from each
immediately ship on cold pack. If
organ collected in sterile
frozen, ship on dry ice.
container
***Fetal Tissue,
At minimum,
Store and ship at room temperature
3
Formalin Fixed
1 cm
section
***Fetal Tissue,
Paraffin embedded block
Store and ship at room temperature
Paraffin Block
Zika
***Fetal Tissue,
As above
Store and ship at room temperature
Histopathology and
Fixed or Paraffin
Immunohistochemistry
Block
* Specimens must be received within 24 hours of collection.
**Do not use glass vacutainer tube for blood collection. Do not use tubes that contain anti-coagulants.
***To optimize evaluation of possible Zika virus infection on fetal tissues, please provide both formalin fixed and unfixed tissues. If it is not possible to
provide both types of tissues, prioritize formalin fixed tissues. For additional information regarding collection of fetal or infant tissues, please contact the
Public Health Laboratories for guidance.
ZIKA VIRUS TESTING AND REPORT FORM AND INSTRUCTIONS - ZikaInfoTestReq (6/23/16)
Page 6 of 6
CONFIDENTIAL – This material is subject to the Official Information Privilege Act
 1
1 2
2 3
3 4
4 5
5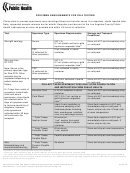 6
6








