Stoichiometry Cheat Sheet
ADVERTISEMENT
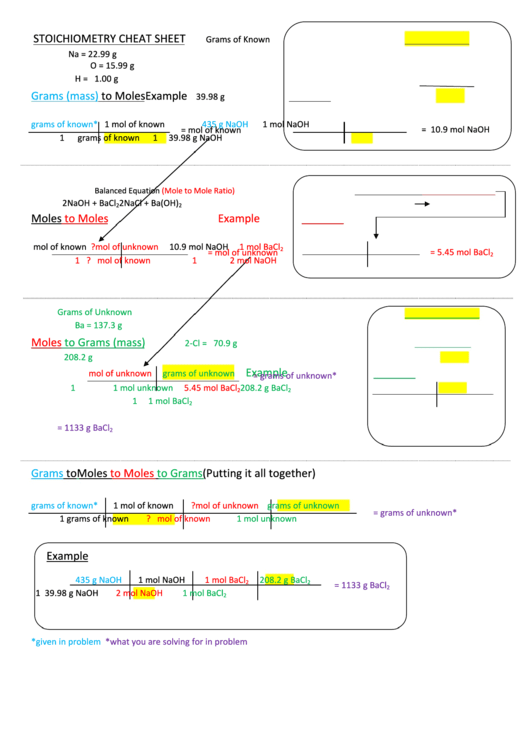
STOICHIOMETRY CHEAT SHEET
Grams of Known
Na = 22.99 g
O = 15.99 g
H = 1.00 g
Grams (mass)
to Moles
Example
39.98 g
grams of known*
1 mol of known
435 g NaOH
1 mol NaOH
= 10.9 mol NaOH
= mol of known
1
grams of known
1
39.98 g NaOH
Balanced Equation
(Mole to Mole Ratio)
2NaOH + BaCl
2NaCl + Ba(OH)
2
2
Moles
to Moles
Example
mol of known
? mol of unknown
10.9 mol NaOH
1 mol BaCl
2
= mol of unknown
= 5.45 mol BaCl
2
1
? mol of known
1
2 mol NaOH
Grams of Unknown
Ba = 137.3 g
Moles
to Grams (mass)
2-Cl = 70.9 g
208.2 g
Example
mol of unknown
grams of unknown
= grams of unknown*
1
1 mol unknown
5.45 mol BaCl
208.2 g BaCl
2
2
1
1 mol BaCl
2
= 1133 g BaCl
2
Grams
to Moles
to Moles
to Grams
(Putting it all together)
grams of known*
1 mol of known
? mol of unknown
grams of unknown
= grams of unknown*
1
grams of known
? mol of known
1 mol unknown
Example
435 g NaOH
1 mol NaOH
1 mol BaCl
208.2 g BaCl
2
2
= 1133 g BaCl
2
1
39.98 g NaOH
2 mol NaOH
1 mol BaCl
2
*given in problem
*what you are solving for in problem
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1