Molecular Geometry And Polarity Worksheet Page 2
ADVERTISEMENT
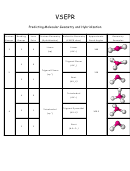
Fill in the table below.
If there is no net dipole moment, under the NET DIPOLE ARROW column, enter the symbol Ø.
-
If a molecule has a dipole moment (arrow), then the molecule is POLAR.
-
If a molecule has no net dipole moment (Ø), then the molecule is NONPOLAR.
NET
IS MOLECULE
3-D STRUCTURE WITH
DIPOLE
FORMULA
LEWIS STRUCTURE
MOLECULAR SHAPE
POLAR OR
DIPOLE MOMENTS
MOMENT
NONPOLAR?
ARROW
ex
CH
Cl
polar
tetrahedral
3
1)
H
S
2
2)
SiO
2
3)
CI
4
4)
NBr
3
5)
CH
O
2
6)
CHI
3
7)
C
H
2
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2