Vsepr And Polarity Worksheet
ADVERTISEMENT
Chemistry 121 Problem set V Solutions - 1
Chem 121 Problem Set V Lewis Structures, VSEPR and Polarity
ANSWERS
1.
Species
Elecronegativity difference in bond
Bond Polarity
Mp
∆E = 3.0 - 3.0 = 0 for N-Cl
NCl
very weakly polar covalent
< -40°C
3
∆E = 3.0 - 1.5 = 1.5 for Cl-Al
AlCl
strongly polar covalent
190°C at 2.5 atm
3
∆E = 3.5 - 2.5 = 1.0 for O-S
SO
polar covalent
-73°C
3
∆E = 2.5 - 0.8 = 1.7 for I-K
KI
ionic (barely)
1330°C
∆E = 2.5 - 2.1 = 0.4 for C-H
CH
weakly polar covalent
-182°C
4
∆E = 4.0 - 2.5 = 1.5 for F-C
CF
strongly polar covalent
-150°C
4
Why does CF
have a similar mp to CH
when the bond polarities are very different?
4
4
2.
N
a) valence electrons = 10
2
b) first structure gives
bp 2 + lp 12 = 14,
10 - 14 = -4 or two extra bonds
c) second structure gives
bp 6 + lp 4 = 10
d) Formal charge(N) = 5 - 3 - 2 = 0
N
N
N N
NO + a) valence electrons = 5 + 6 - 1 = 10
b) first structure gives
bp 2 + lp 12 = 14,
10 - 14 = -4 or two extra bonds
c) second structure gives
bp 6 + lp 4 = 10
d) FC(N) = 5 - 3- 2 = 0 ,
FC(O) = 6 - 3 - 2 = +1
N
O
N
O
O
a) valence electrons = 12
2
b) first structure gives
bp 2 + lp 12 = 14,
12 - 14 = -2 or one extra bond
c) second structure gives bp 4 + lp 8 = 12
d) FC(O) = 6 - 2 - 4 = 0
O
O
O
O
-
ClO
a) valence electrons = 7 + 18 + 1 = 26
3
b) first structure gives
bp 6 + lp 20 = 26,
26 - 26 = 0 ok
c) second structure has FC (O) = 6 - 1 - 6 = -1 FC (Cl) = 7 - 3 - 2 = +2
O
O
O
2
Cl
Cl
Cl
O
O
O
O
O
O
O
O
O
Cl
Cl
Cl
O
O
O
O
O
O
Cl
a) valence electrons = 14
2
b) first structure gives
bp 2 + lp 12 = 14,
14 - 14 = 0 ok
c) FC(Cl) = 7 - 1 - 6 = 0
Cl
Cl
XeO
a) valence electrons = 8 + 18 = 26
3
b) first structure gives
bp 6 + lp 20 = 26,
26 - 26 = 0 ok
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1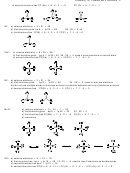 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10








