Chemistry Reference Tables - Ncdpi, 2008 Page 3
ADVERTISEMENT
Formulas
m
=
D
D = density
V
= ° +
K
C 273
m = mass
PV
P V
=
1 1
2 2
V = volume
T
T
1
2
=
+
+
+
P
P
P
P
...
K = Kelvin
t
1
2
3
=
M V
M V
P = pressure
1 1
2 2
=
PV
nRT
R = gas constant
moles of solute
M =
T = temperature
liter of solution
=
Δ
q
mC T
M = molarity
p
=
q
mH
n = number of moles
v
=
q
mH
q = quantity of heat energy
f
+
=
C = specific heat
pH
pOH
14
p
+
= −
H = heat of vaporization
pH
log[H ]
v
= −
−
H = heat of fusion
pOH
log[OH ]
f
K = equilibrium constant for
w
+
−
−
=
= ×
14
K
[H ][OH ]
1 10
w
the ionization of water
+
−
=
pH
[H ]
10
−
=
−
pOH
[OH ]
10
NCDPI Reference Tables for Chemistry (October 2008)
Page 3
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1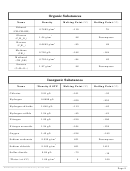 2
2 3
3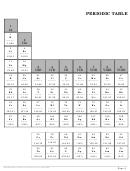 4
4 5
5 6
6 7
7 8
8








