Chemistry Reference Tables - Ncdpi, 2008 Page 6
ADVERTISEMENT
SOLUBILITY RULES
Soluble:
Insoluble (0.10 M or greater):
•
•
All Nitrates, Acetates, Ammonium,
All Carbonates and Phosphates
and Group 1 (IA) salts
except Group 1 (IA) and Ammonium
•
All Chlorides, Bromides, and Iodides,
•
All Hydroxides except Group 1 (IA),
except Silver, Lead, and Mercury(I)
Strontium, Barium, and
•
All Fluorides except Group 2 (IIA),
Ammonium
Lead(II), and Iron(III)
•
All Sulfides except Group 1 (IA),
•
All Sulfates except Calcium,
2 (IIA), and Ammonium
Strontium, Barium, Mercury,
•
All Oxides except Group 1 (IA)
Lead(II), and Silver
Guidelines for Predicting the Products of Selected Types of Chemical Reactions
Key: M = Metal
NM = Nonmetal
1.
SYNTHESIS:
a. Formation of binary compound: A + B → AB
O → base
b. Metal oxide and water: MO + H
2
O → acid
c. Nonmetal oxide and water: (NM)O + H
2
2.
DECOMPOSITION:
a. Binary compounds: AB → A + B
→ MO + CO
b. Metallic carbonates: MCO
3
2
→ MCO
c. Metallic hydrogen carbonates: MHCO
( s ) + H
O ( l ) + CO
( g )
3
3
2
2
d. Metallic hydroxides: MOH → MO + H
O
2
→ MCl + O
e. Metallic chlorates: MClO
3
2
f. Oxyacids decompose to nonmetal oxides and water: acid → (NM)O + H
O
2
3.
SINGLE REPLACEMENT:
a. Metal-Metal replacement: A + BC → AC + B
O → MOH + H
b. Active metal replaces H from water: M + H
2
2
c. Active metal replaces H from acid: M + HX → MX + H
2
d. Halide-Halide replacement: D + BC → BD + C
DOUBLE REPLACEMENT: AB + CD → AD + CB
4.
a. Formation of a precipitate from solution
b. Acid-Base neutralization
5.
COMBUSTION REACTION
Hydrocarbon + oxygen → carbon dioxide + water
NCDPI Reference Tables for Chemistry (October 2008)
Page 6
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1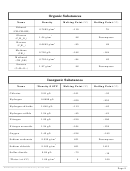 2
2 3
3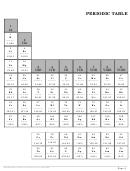 4
4 5
5 6
6 7
7 8
8








