Chem1612 2014-N-12 Worksheet With Answers - The University Of Sydney - 2014 Page 13
ADVERTISEMENT
CHEM1612
2009-N-16
November 2009
Marks
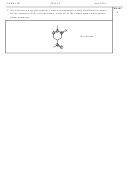
What is the value of the equilibrium constant for the following reaction at 298 K?
•
3
3+
2+
2Fe
(aq) + 3Sn(s) → 2Fe(s) + 3Sn
(aq)
Relevant electrode potentials can be found on the data page.
The relevant reduction potentials are:
3+
-
Fe
(aq) + 3e
à Fe(s)
E° = -0.04 V
2+
-
Sn
(aq) + 2e
à Sn(s)
E° = -0.14 V
2+
As the Sn
/ Sn couple is the more negative, it is reversed giving:
E° = (-0.04 + 0.14) V = 0.10 V
The equilibrium constant, K, is related to the standard reduction potential using:
E° = (RT/nF) × lnK
lnK = nFE° / RT = (6 × 96485 × 0.10) / (8.314 × 298) = 23.37
23.37
10
K = e
= 1.4 × 10
10
Answer: 1.4 × 10
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28