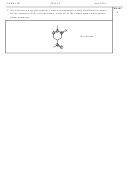
Chem1612 2014-N-12 Worksheet With Answers - The University Of Sydney - 2014 Page 7
ADVERTISEMENT
CHEM1612
2012-N-11
November 2012
Calculate the standard Gibbs free energy of the reaction at 25 °C.
The Gibbs free energy change is related to the standard cell potential through:
o
o
= -nFE
ΔG
-1
-1
= - 6 × (96485 mol
) × (0.50 V) = -290 kJ mol
-1
Answer: -290 kJ mol
Express the overall reaction in the shorthand voltaic cell notation.
3+
2+
Cr(s) | Cr
(aq) || Ni
(aq) | Ni(s)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28