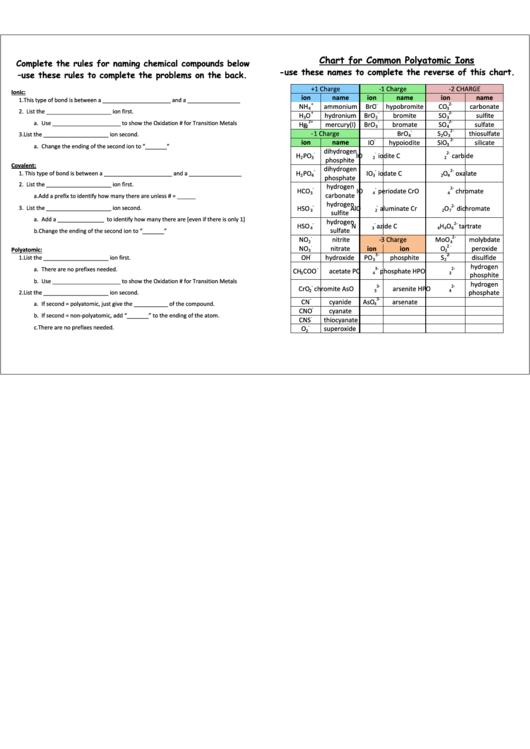
Chart For Common Polyatomic Ions
ADVERTISEMENT
Chart for Common Polyatomic Ions
Complete the rules for naming chemical compounds below
-use these names to complete the reverse of this chart.
–use these rules to complete the problems on the back.
+1 Charge
-1 Charge
-2 CHARGE
Ionic:
ion
name
ion
name
ion
name
1. This type of bond is between a ______________________ and a _________________
+
-
2-
NH
ammonium
BrO
hypobromite
CO
carbonate
4
3
2. List the _____________________ ion first.
+
-
2-
H
O
hydronium
BrO
bromite
SO
sulfite
3
2
3
2+
-
2-
a. Use ______________________ to show the Oxidation # for Transition Metals
Hg
mercury(I)
BrO
bromate
SO
sulfate
2
3
4
-
2-
-1 Charge
BrO
S
O
thiosulfate
3. List the _____________________ ion second.
4
2
3
-
2-
IO
hypoiodite
SiO
silicate
ion
name
3
a. Change the ending of the second ion to “_______”
dihydrogen
-
-
2-
H
PO
IO
iodite
C
carbide
2
3
2
2
phosphite
Covalent:
dihydrogen
-
-
2-
1. This type of bond is between a ______________________ and a _________________
H
PO
IO
iodate
C
O
oxalate
2
4
3
2
4
phosphate
2. List the _____________________ ion first.
hydrogen
-
-
2-
HCO
IO
periodate
CrO
chromate
3
4
4
carbonate
a. Add a prefix to identify how many there are unless # = ______
hydrogen
-
-
2-
3. List the _____________________ ion second.
HSO
AlO
aluminate
Cr
O
dichromate
3
2
2
7
sulfite
a. Add a _______________ to identify how many there are [even if there is only 1]
hydrogen
-
-
2-
HSO
N
azide
C
H
O
tartrate
4
3
4
4
6
sulfate
b. Change the ending of the second ion to “_______”
-
2-
NO
nitrite
-3 Charge
MoO
molybdate
2
4
-
2-
NO
nitrate
ion
ion
O
peroxide
Polyatomic:
3
2
-
3-
2-
OH
hydroxide
PO
phosphite
S
disulfide
1. List the _____________________ ion first.
3
2
hydrogen
-
3-
2-
a. There are no prefixes needed.
CH
COO
acetate
PO
phosphate
HPO
3
4
3
phosphite
b. Use ______________________ to show the Oxidation # for Transition Metals
hydrogen
-
3-
2-
CrO
chromite
AsO
arsenite
HPO
2
3
4
2. List the _____________________ ion second.
phosphate
-
3-
CN
cyanide
AsO
arsenate
a. If second = polyatomic, just give the ___________ of the compound.
4
-
CNO
cyanate
b. If second = non-polyatomic, add “_______” to the ending of the atom.
-
CNS
thiocyanate
c. There are no prefixes needed.
-
O
superoxide
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








