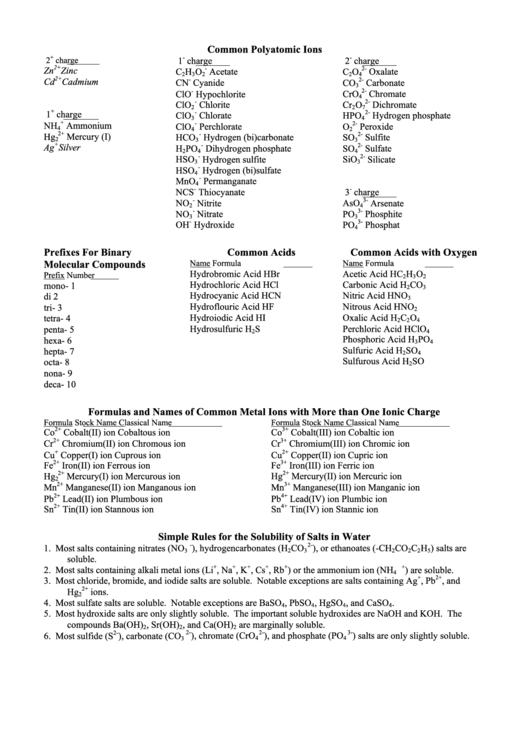
Common Polyatomic Ions Chart
ADVERTISEMENT
Common Polyatomic Ions
+
-
-
2
charge
1
charge
2
charge
2+
-
2-
Zn
Zinc
C
H
O
Acetate
C
O
Oxalate
2
3
2
2
4
2+
-
2-
Cd
Cadmium
CN
Cyanide
CO
Carbonate
3
-
2-
ClO
Hypochlorite
CrO
Chromate
4
-
2-
ClO
Chlorite
Cr
O
Dichromate
2
2
7
+
-
2-
1
charge
ClO
Chlorate
HPO
Hydrogen phosphate
3
4
+
-
2-
NH
Ammonium
ClO
Perchlorate
O
Peroxide
4
4
2
2+
-
2-
Hg
Mercury (I)
HCO
Hydrogen (bi)carbonate
SO
Sulfite
2
3
3
+
-
2-
Ag
Silver
H
PO
Dihydrogen phosphate
SO
Sulfate
2
4
4
-
2-
HSO
Hydrogen sulfite
SiO
Silicate
3
3
-
HSO
Hydrogen (bi)sulfate
4
-
MnO
Permanganate
4
-
-
NCS
Thiocyanate
3
charge
-
3-
NO
Nitrite
AsO
Arsenate
2
4
-
3-
NO
Nitrate
PO
Phosphite
3
3
-
3-
OH
Hydroxide
PO
Phosphat
4
Prefixes For Binary
Common Acids
Common Acids with Oxygen
Molecular Compounds
Name
Formula
Name
Formula
Hydrobromic Acid
HBr
Acetic Acid
HC
H
O
Prefix
Number
2
3
2
Hydrochloric Acid
HCl
Carbonic Acid
H
CO
mono-
1
2
3
Hydrocyanic Acid
HCN
Nitric Acid
HNO
di
2
3
Hydroflouric Acid
HF
Nitrous Acid
HNO
tri-
3
2
Hydroiodic Acid
HI
Oxalic Acid
H
C
O
tetra-
4
2
2
4
Hydrosulfuric
H
S
Perchloric Acid
HClO
penta-
5
2
4
Phosphoric Acid
H
PO
hexa-
6
3
4
Sulfuric Acid
H
SO
hepta-
7
2
4
Sulfurous Acid
H
SO
octa-
8
2
nona-
9
deca-
10
Formulas and Names of Common Metal Ions with More than One Ionic Charge
Formula
Stock Name
Classical Name
Formula
Stock Name
Classical Name
2+
3+
Co
Cobalt(II) ion
Cobaltous ion
Co
Cobalt(III) ion
Cobaltic ion
2+
3+
Cr
Chromium(II) ion
Chromous ion
Cr
Chromium(III) ion
Chromic ion
+
2+
Cu
Copper(I) ion
Cuprous ion
Cu
Copper(II) ion
Cupric ion
2+
3+
Fe
Iron(II) ion
Ferrous ion
Fe
Iron(III) ion
Ferric ion
2+
2+
Hg
Mercury(I) ion
Mercurous ion
Hg
Mercury(II) ion
Mercuric ion
2
2+
3+
Mn
Manganese(II) ion
Manganous ion
Mn
Manganese(III) ion
Manganic ion
2+
4+
Pb
Lead(II) ion
Plumbous ion
Pb
Lead(IV) ion
Plumbic ion
2+
4+
Sn
Tin(II) ion
Stannous ion
Sn
Tin(IV) ion
Stannic ion
Simple Rules for the Solubility of Salts in Water
-
2-
1. Most salts containing nitrates (NO
), hydrogencarbonates (H
CO
), or ethanoates (-CH
CO
C
H
) salts are
3
2
3
2
2
2
5
soluble.
+
+
+
+
+
+
2. Most salts containing alkali metal ions (Li
, Na
, K
, Cs
, Rb
) or the ammonium ion (NH
) are soluble.
4
+
2+
3. Most chloride, bromide, and iodide salts are soluble. Notable exceptions are salts containing Ag
, Pb
, and
2+
Hg
ions.
2
4. Most sulfate salts are soluble. Notable exceptions are BaSO
, PbSO
, HgSO
, and CaSO
.
4
4
4
4
5. Most hydroxide salts are only slightly soluble. The important soluble hydroxides are NaOH and KOH. The
compounds Ba(OH)
, Sr(OH)
, and Ca(OH)
are marginally soluble.
2
2
2
2-
2-
2-
3-
6. Most sulfide (S
), carbonate (CO
), chromate (CrO
), and phosphate (PO
) salts are only slightly soluble.
3
4
4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








