Further Ionisation Energies
ADVERTISEMENT
C h e m g u id e – a n s w e r s
FURTHER IONISATION ENERGIES
1.
Na
+
Na
2+
+ e
-
(g)
(g)
The equation must include the state symbols.
2. Beryllium's electronic structure is 1s
2
2s
2
. The first two electrons to be removed are coming from
the 2s orbital which is screened from the nucleus by the 1s electrons. The third electron is being
removed from the 1s orbital which is much closer to the nucleus, and has no screening.
3. Look for the first big jump in ionisation energy. This will occur when the electron is removed from
an inner level. The number of electrons removed before the jump is the same as the group number.
a) Group 3
b) Group 2
c) Group 6
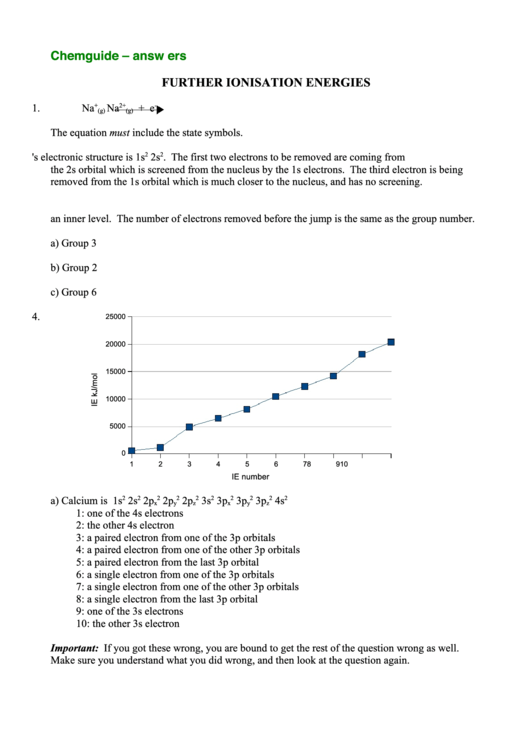
4.
25000
20000
15000
10000
5000
0
1
2
3
4
5
6
7
8
9
10
IE number
a) Calcium is 1s
2
2s
2
2p
2
2p
2
2p
2
3s
2
3p
2
3p
2
3p
2
4s
2
x
y
z
x
y
z
1: one of the 4s electrons
2: the other 4s electron
3: a paired electron from one of the 3p orbitals
4: a paired electron from one of the other 3p orbitals
5: a paired electron from the last 3p orbital
6: a single electron from one of the 3p orbitals
7: a single electron from one of the other 3p orbitals
8: a single electron from the last 3p orbital
9: one of the 3s electrons
10: the other 3s electron
Important: If you got these wrong, you are bound to get the rest of the question wrong as well.
Make sure you understand what you did wrong, and then look at the question again.
ADVERTISEMENT
0 votes
 1
1 2
2 3
3








