Questions On Successive Ionisation Energies
ADVERTISEMENT
C h e m g u id e – q u e s t i o n s
FURTHER IONISATION ENERGIES
You may need a copy of the Periodic Table for some of these questions.
1. The second ionisation energy of sodium is 4560 kJ mol
-1
. Write the equation which represents the
reaction occurring during the second ionisation energy of sodium.
2. The four successive ionisation energies of beryllium are: 900 1760 14800 21000. Why is there
a very big increase in size between the second and third ionisation energies?
3. The following are all lists of successive ionisation energies for different elements. In each case,
decide which group of the Periodic Table the element is to be found in. None of these elements
comes from the d-block.
a) 799 2420 3660 25000 32800
b) 736 1450 7740 10500 13600 18000 21700 25600
c) 1000 2260 3390 4540 6990 8490 27100 31700
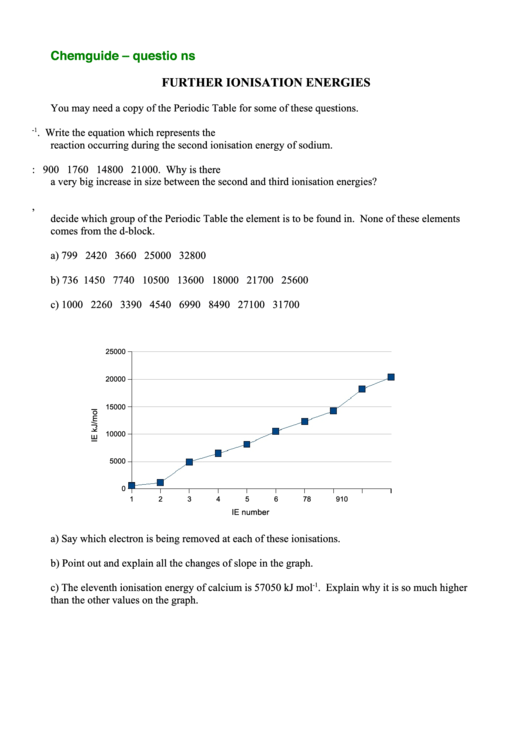
4. The graph shows the first ten ionisation energies for calcium.
25000
20000
15000
10000
5000
0
1
2
3
4
5
6
7
8
9
10
IE number
a) Say which electron is being removed at each of these ionisations.
b) Point out and explain all the changes of slope in the graph.
c) The eleventh ionisation energy of calcium is 57050 kJ mol
-1
. Explain why it is so much higher
than the other values on the graph.
ADVERTISEMENT
0 votes
 1
1 2
2








