Periodic Properties Worksheet Key
ADVERTISEMENT
1
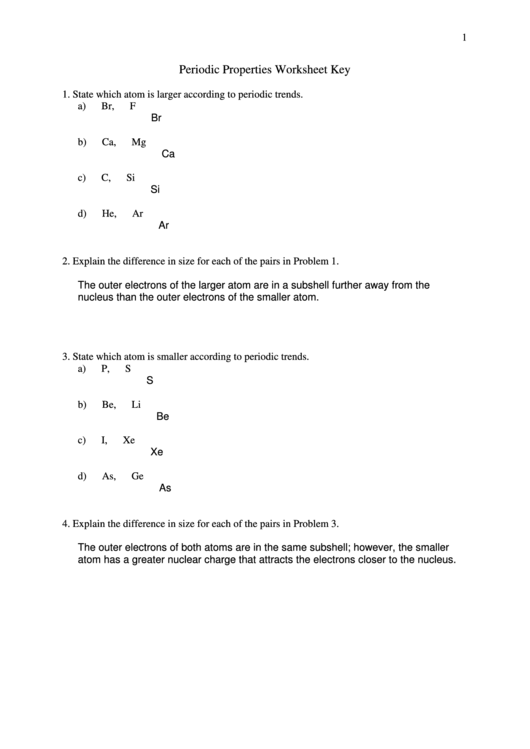
Periodic Properties Worksheet Key
1. State which atom is larger according to periodic trends.
a) Br, F
Br
b) Ca, Mg
Ca
c) C, Si
Si
d) He, Ar
Ar
2. Explain the difference in size for each of the pairs in Problem 1.
The outer electrons of the larger atom are in a subshell further away from the
nucleus than the outer electrons of the smaller atom.
3. State which atom is smaller according to periodic trends.
a) P, S
S
b) Be, Li
Be
c) I, Xe
Xe
d) As, Ge
As
4. Explain the difference in size for each of the pairs in Problem 3.
The outer electrons of both atoms are in the same subshell; however, the smaller
atom has a greater nuclear charge that attracts the electrons closer to the nucleus.
ADVERTISEMENT
0 votes
 1
1 2
2 3
3 4
4








