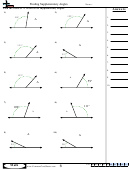
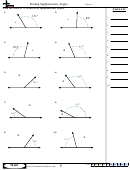
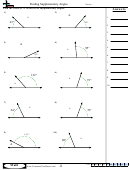
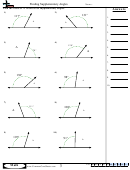
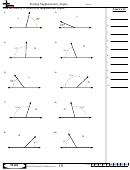
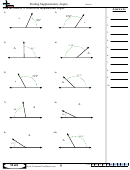
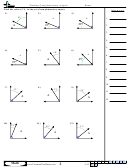
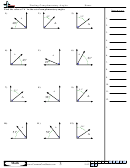
Finding Bond Angles Shapes And Hybridizations Page 2
ADVERTISEMENT
Linear: The atoms in the molecule are in a straight line. This can be either because there are
only two atoms in the molecule (in which case there is no bond angle, as there need to be
three atoms to get a bond angle) or because the three atoms are lined up in a straight line
(corresponding to a 180 degree bond angle).
There are other types, but we won't worry about them.
Using a flow chart to figure out what hybridization, shape, and bond angle an atom
has
Take a look at this flow chart. I'll explain how to use it to find all the stuff above at the end.
Complicated, huh? Here's how to use it:
1) Draw the Lewis structure for the molecule. This vital if you're going to get the answer right.
2) Count the number of "things" on the atom you're interested in. Let's say that you're looking
at methane, CH
. If you want to find the bond angles, shape, and hybridization for carbon, count the
4
number of things that are stuck to it.
Now, the vague term "things" refers to atoms and lone pairs. IT DOES NOT REFER TO THE
NUMBER OF BONDS! When you look at methane, there are four atoms stuck to it, so you'd go down
the line that says "four" toward the green boxes on this chart.
People get confused with multiple bonds. Take carbon dioxide, for example. There are four bonds
(carbon is double-bonded to each oxygen) but only two oxygen atoms bonded to carbon. In this
case, we count two things stuck to carbon, because we only count the atoms, NOT the number of
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3