Finding Bond Angles Shapes And Hybridizations Page 3
ADVERTISEMENT
bonds.
Likewise, with ammonia there are four things. Three of the things on nitrogen are hydrogen atoms
and the fourth is a lone pair. For the purposes of VSEPR, lone pairs count exactly the same as
atoms, because they consist of negative charge, too.
3) Count the number of lone pairs that are on the atom you're interested in. IMPORTANT:
This does NOT mean to count the number of lone pairs on all of the atoms in the molecule. Lone
pairs on other atoms aren't important - what's important is only what's directly stuck to the atom you're
interested in.
We mentioned above that methane has four things stuck to it. Since all four things are hydrogen
atoms, we moved toward the green boxes on the flow chart. When we get to our second question,
we find that there are no lone pairs on carbon, so our answer is zero. When we go down the line that
3
says "zero" from that box, we find that methane is sp
hybridized, with a 109.5 degree bond angle and
tetrahedral shape.
And, hey, that's what we were looking for!
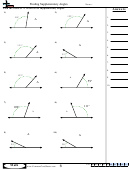
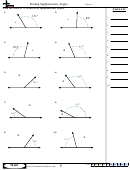
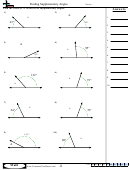
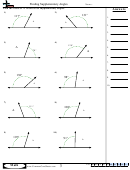
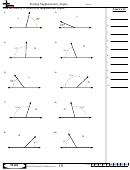
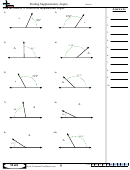
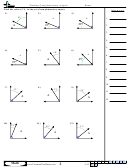
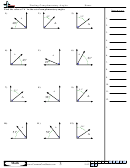
Some sample problems:
What are the shapes, bond angles, and hybridizations of the following molecules? Use the flow chart
and instructions above to figure it out.
1) carbon tetrabromide
2) phosphorus trichloride
3) oxygen
4) the chlorine atom in hydrochloric acid (HCl)
5) boron trichloride
6) CH
O
2
7) sulfur difluoride
8) either carbon atom in C
H
2
2
The answers are below:
3
1) sp
, tetrahedral, 109.5 degrees.
3
2) sp
, trigonal pyramidal, 107.5 degrees.
2
3) sp
, linear, no bond angle
3
4) sp
, linear, no bond angle
2
5) sp
, trigonal planar, 120 degrees
2
6) sp
, trigonal planar, 120 degrees
3
7) sp
, bent, 104.5 degrees
8) sp, linear, 180 degrees
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3