Protonation Of Polyaniline With Lightly Sulfonated Polystyrene
ADVERTISEMENT
ELSEVIER
Synthetic
Metals
84 (1997)
103-104
Protonation of polyaniline with lightly sulfonated polystyrene
Yueping Fu, R. A. Weiss
Polymer Science Program and Department
of Chemical Engineering
UniversiQ
of Connecticut,
Storrs, CT 06269-3136,
UsA
Abstract
Protonation
of polyaniline
base with lightly sulfonated polystyrene in polar solvents such as dimethyl
sulfoxide
(DMSO)
and
N-methyl
pyrrolidone
(NMP)
was investigated
by UV-Vis
absorption
spectroscopy.
The isosbestic
point clearly
shows that
quinoid
unit and semiquinoid
unit are in equilibrium
and is a function
of the sulfonic
acid concentration.
The protonation
of
polyaniline
is retarded in NMP compared to DMSO due to prevalent hydrogen bonding.
keywords:
Polyaniline,
sulfonated
polystyrene,
UV-Vis
absorption
1. Introduction
Polyaniline
(PANI)
has very promising
industrial
appli-
cations because of its good environmental
stability and facile
synthesis
[I, 21. Polyaniline
doped
with
organic
protonic
acids [3], and polymeric
acid [4] shows improved
solubility
and processibility.
We are interested in preparing
conductive polymer blends
with improved
processability
and better controlled
conduc-
tivity.
Theoretically,
the conductivity
of polyaniline
can be
varied from lo-lo
to 10 S/cm as a function of doping level. In
reality, the conductivity
of polyaniline
increases dramatically
at low doping level and levels off at ca. 10% doping [.5]. Here,
lightly
sulfonated
polystyrene
(HSPS)
was chosen
as the
polymer
matrix,
because the randomly
placed sulfonic
acid
groups on the polystyrene
chains behave as a dilute acid.
It
will
protonate
the imine nitrogen
sites of polyaniline,
while
possibly
retaining
the processability
of polystyrene.
This
molecular
protonation
should promote
compatibility
between
polyaniline
and
polystyrene
within
the
blends,
and
simultaneously
transform
the insulating
polyaniline
base
form to the metallic
conducting
form, thereby rendering
the
blends conductive.
The use of polymeric
dopants may signi-
ficantly improve
the stability of the resulting
polymer blends,
because small molecule
dopants
tend to migrate
out of the
polymer
matrix.
In this paper, we present direct evidence of doping PAN1
through
protonation
by sulfonic
acid groups of lightly
sulfo-
nated polystyrene
in solutions
and its dependence
on the
solvent environment.
2.
Experimental
PANI was synthesized
by the oxidative
polymerization
of aniline
in
1.0
M
aqueous
HCl
with
ammonium
peroxydisulfate
(APS) as oxidant, as described previously
[6].
Polystyrene
(M, = 100,000, M,
= 280,000) was sulfonated to
5.3 mol % in dichloroethane
at 50 OC with
acetyl sulfate
following
the procedure
of Makowski
et al [7].
PANI/HSPS
solutions
with
different
molar ratios were
prepared
by mixing
appropriate
volumes of two solutions,
1
mM PAN1 base (based on the approximate
aniline
repeat unit
-CgHqNH-
) in DMSO or NMP and 10 mM HSPS (based on
0379-6779/97/%17.00
0 1997
Elswier
Science
S.A. All
rights
reserved
PII SO3794779(96)03857-X
styrene unit) in the same solvent, to give a clear solution,
keeping the concentration
of PANI constant at 0.06 mM. UV-
Vis absorbance spectra were recorded on Perkin-Elmer
Lambda
6 UV/VIS
spectrophotometer.
3. Results
and
discussion
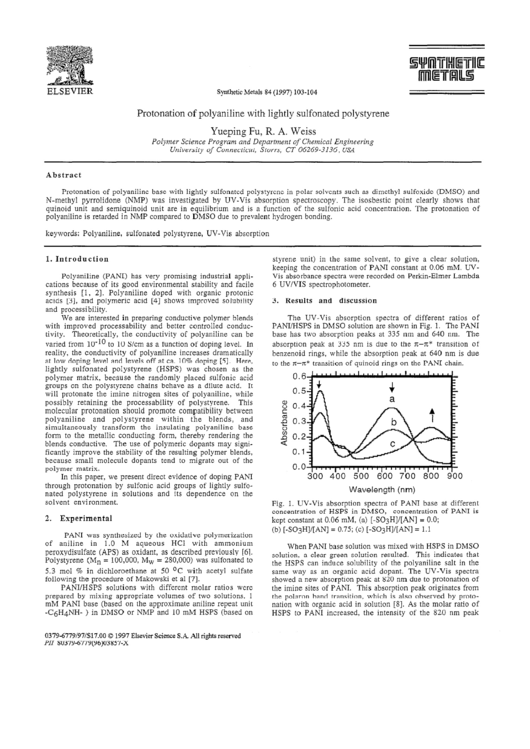
The UV-Vis
absorption
spectra of different
ratios
of
PANI/HSPS
in DMSO solution are shown in Fig. 1. The PANI
base has two absorption
peaks at 335 nm and 640 nm.
The
absorption
peak at 335 nm is due to the rc-TC* transition
of
benzenoid
rings, while the absorption
peak at 640 nm is due
to the X-Z*
transition
of quinoid rings on the PAN1 chain.
“I
‘II
-I’
I”
I”
“I
‘*.
I-’
“I
0.5
g 0.4
B
5
0.3
JJ 0.2
0.1
0.0
300
400
500
600
700
800
900
Wavelength
(nm)
Fig. 1. UV-Vis
absorption
spectra of PANI base at different
concentration
of HSPS in DMSO,
concentration
of PAN1 is
kept constant at 0.06 mM, (a) [-S03H]/[AN]
= 0.0;
(b) [-S03H]/[AN]
= 0.75; (c) [-S03H]/[AN]
= 1.1
When PAN1 base solution was mixed with HSPS in DMSO
solution,
a clear green solution
resulted.
This indicates
that
the HSPS can induce solubility
of the polyaniline
salt in the
same way as an organic
acid dopant.
The UV-Vis
spectra
showed a new absorption peak at 820 nm due to protonation
of
the imine sites of PANI.
This absorption
peak originates
from
the polaron
band transition,
which is also observed by proto-
nation with organic acid in solution
[8]. As the molar ratio of
HSPS to PAN1 increased,
the intensity
of the 820 nm peak
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Business
 1
1 2
2








