Ion Chart Chemistry Paper
ADVERTISEMENT
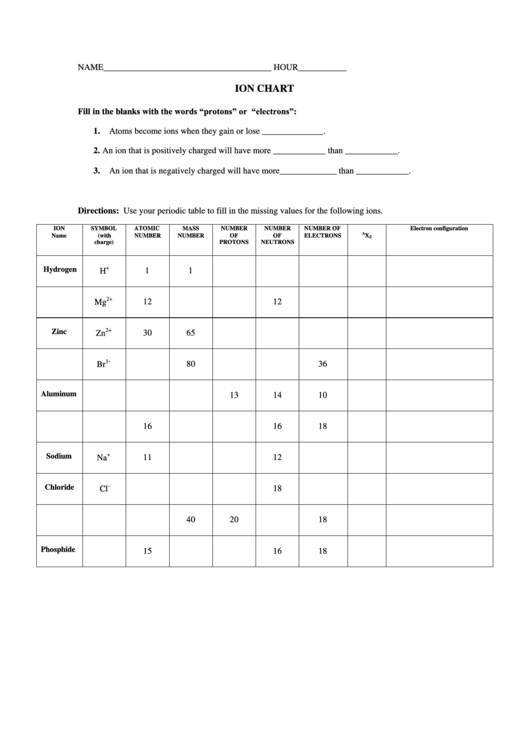
NAME______________________________________
HOUR___________
ION CHART
Fill in the blanks with the words “protons” or “electrons”:
1.
Atoms become ions when they gain or lose ______________.
2.
An ion that is positively charged will have more ____________ than ____________.
3.
An ion that is negatively charged will have more_____________ than ____________.
Directions: Use your periodic table to fill in the missing values for the following ions.
ION
SYMBOL
ATOMIC
MASS
NUMBER
NUMBER
NUMBER OF
Electron configuration
A
Name
(with
NUMBER
NUMBER
OF
OF
ELECTRONS
X
Z
charge)
PROTONS
NEUTRONS
+
Hydrogen
H
1
1
2+
Mg
12
12
2+
Zinc
Zn
30
65
1-
Br
80
36
Aluminum
13
14
10
16
16
18
+
Sodium
Na
11
12
-
Chloride
Cl
18
40
20
18
Phosphide
15
16
18
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








