Aqa Chemistry Mechanism Summary Page 4
ADVERTISEMENT
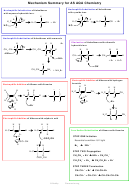
KOH aqueous
heat under reflux
Nu Sub
dihaloalkane
diol
poly(alkene)
high pressure
Br
Cl
room temp
2,
2
catalyst
EAdd
alkane
alkene
Br
Cl
2,
2
Step 1 H
SO
2
4
UV light
EAdd
Fr Sub
conc. H
SO
or
2
4
Step 2 H
O warm
2
conc. H
PO
3
4
hydrolysis
Elimination,
dehydration
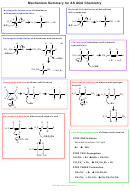
KOH aqueous
heat under reflux
NuSub
haloalkane
alcohol
NaBH
4
NaBH
Alcoholic NH
4
3
Red Nu Add
KCN in ethanol/
Red Nu Add
heat under
water mixture heat
pressure
under reflux
NuSub
NuSub
If secondary
If primary
+
Na
Cr
O
/H
LiAlH
in ether
o
2
2
7
1
amine
nitrile
4
+
Na
Cr
O
/H
heat
reduction
2
2
7
heat and distill
oxidation
haloalkane
partial ox
Caboxylic acid +
NuSub
H
SO
2
4
heat
ketone
aldehyde
o
2
amine
esterification
Acyl chloride
room temp
o
3
amine
Nu add/elim
NaCN + H
SO
Quaternary salt
2
4
Nu Add
+
(If primary) Na
Cr
O
/H
secondary
2
2
7
Esters and
heat under reflux + excess
hydroxynitrile
amides can be
amide
oxidising agent
hydrolysed by
Oxidation
NaOH and acids
ester
o
Alcohol + H
SO
1
amine
2
4
carboxylic acid
heat
room temp
esterification
Nu add/elim
Alcohol
Primary
room temp
Nu add/elim
H
O room temp
amide
2
Nu add/elim
Acyl chloride/
NH
room temp
3
Nu add/elim
acid anhydride
N Goalby
4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1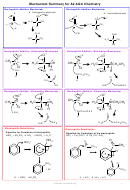 2
2 3
3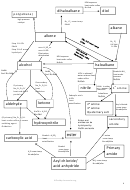 4
4