Energy Profiles Test Asnwers
ADVERTISEMENT
C h e m g u i d e – a n s w e r s
ENERGY PROFILES
1. The lone pair of electrons on the oxygen atom moves towards the slightly positive carbon atom,
starting to form a bond with it. At the same time, the bonding pair of electrons between the carbon
and bromine are repelled towards the bromine, beginning to break that bond. Eventually, a new
bond is made between the carbon and oxygen, and the one between the carbon and bromine is fully
broken.
At one point there is a half-way stage where the bonds are half-broken and half-made. This is
known as the transition state and is the point where the system has its maximum energy, and is most
unstable in energetic terms. It corresponds to the top of the activation energy barrier. The tiniest
electron movement will now tip the system either to go forward to give the products, or to fall back
to give the original reactants again. There is nothing to stop this happening, and so it is impossible
to isolate the transition state.
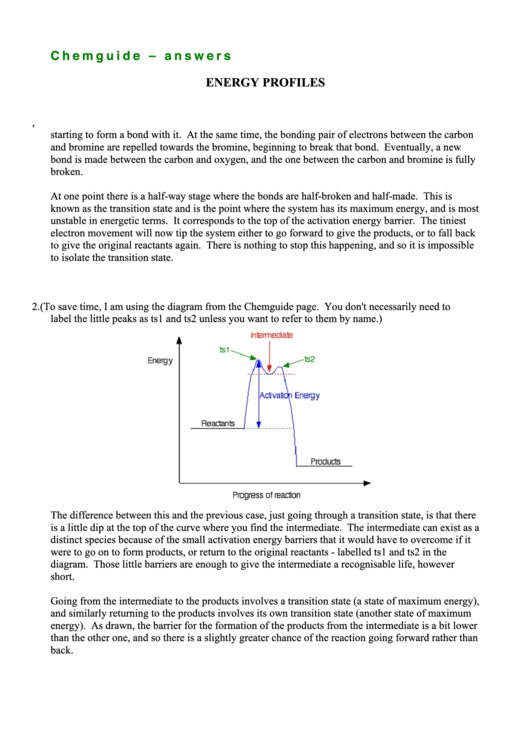
2. (To save time, I am using the diagram from the Chemguide page. You don't necessarily need to
label the little peaks as ts1 and ts2 unless you want to refer to them by name.)
The difference between this and the previous case, just going through a transition state, is that there
is a little dip at the top of the curve where you find the intermediate. The intermediate can exist as a
distinct species because of the small activation energy barriers that it would have to overcome if it
were to go on to form products, or return to the original reactants - labelled ts1 and ts2 in the
diagram. Those little barriers are enough to give the intermediate a recognisable life, however
short.
Going from the intermediate to the products involves a transition state (a state of maximum energy),
and similarly returning to the products involves its own transition state (another state of maximum
energy). As drawn, the barrier for the formation of the products from the intermediate is a bit lower
than the other one, and so there is a slightly greater chance of the reaction going forward rather than
back.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








