Polyatomic Ions Reference Sheet
ADVERTISEMENT
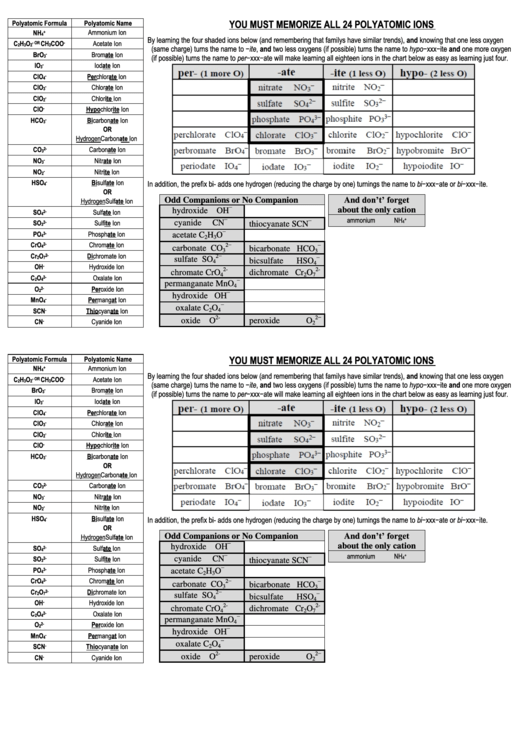
YOU MUST MEMORIZE ALL 24 POLYATOMIC IONS
Polyatomic Formula
Polyatomic Name
NH
Ammonium Ion
+
4
By learning the four shaded ions below (and remembering that familys have similar trends), and knowing that one less oxygen
C
H
O
CH
COO
Acetate Ion
2 - OR
-
2
3
3
(same charge) turns the name to −ite, and two less oxygens (if possible) turns the name to hypo−xxx−ite and one more oxygen
BrO
Bromate Ion
3 -
(if possible) turns the name to per−xxx−ate will make learning all eighteen ions in the chart below as easy as learning just four.
IO
Iodate Ion
3 -
ClO
Perchlorate Ion
4 -
ClO
Chlorate Ion
-
3
ClO
Chlorite Ion
-
2
ClO
Hypochlorite Ion
-
HCO
Bicarbonate Ion
3 -
OR
Hydrogen Carbonate Ion
CO
Carbonate Ion
2-
3
NO
Nitrate Ion
3 -
NO
Nitrite Ion
2 -
HSO
Bisulfate Ion
4 -
In addition, the prefix bi- adds one hydrogen (reducing the charge by one) turnings the name to bi−xxx−ate or bi−xxx−ite.
OR
And don’t’ forget
Odd Companions or No Companion
Hydrogen Sulfate Ion
−
about the only cation
SO
Sulfate Ion
4 2-
hydroxide OH
ammonium
NH
4 +
−
−
SO
Sulfite Ion
2-
cyanide
CN
thiocyanate
SCN
3
PO
Phosphate Ion
−
4 3-
acetate C
H
O
2
3
CrO
Chromate Ion
4 2-
−
2−
carbonate CO
bicarbonate HCO
3
3
Cr
O
Dichromate Ion
7 2-
2−
−
2
sulfate
SO
bicsulfate
HSO
4
4
OH
Hydroxide Ion
-
2-
2-
chromate CrO
dichromate Cr
O
4
2
7
C
O
Oxalate Ion
2-
2
4
−
permanganate MnO
O
Peroxide Ion
4
2 2-
−
hydroxide OH
MnO
Permangat Ion
4 -
−
oxalate C
O
SCN
Thiocyanate Ion
-
2
4
2-
2−
CN
Cyanide Ion
oxide O
peroxide
O
-
2
YOU MUST MEMORIZE ALL 24 POLYATOMIC IONS
Polyatomic Formula
Polyatomic Name
NH
Ammonium Ion
4 +
By learning the four shaded ions below (and remembering that familys have similar trends), and knowing that one less oxygen
C
H
O
CH
COO
Acetate Ion
2 - OR
-
2
3
3
(same charge) turns the name to −ite, and two less oxygens (if possible) turns the name to hypo−xxx−ite and one more oxygen
BrO
Bromate Ion
3 -
(if possible) turns the name to per−xxx−ate will make learning all eighteen ions in the chart below as easy as learning just four.
IO
Iodate Ion
-
3
ClO
Perchlorate Ion
4 -
ClO
Chlorate Ion
3 -
ClO
Chlorite Ion
2 -
ClO
Hypochlorite Ion
-
HCO
Bicarbonate Ion
-
3
OR
Hydrogen Carbonate Ion
CO
Carbonate Ion
3 2-
NO
Nitrate Ion
3 -
NO
Nitrite Ion
2 -
HSO
Bisulfate Ion
-
In addition, the prefix bi- adds one hydrogen (reducing the charge by one) turnings the name to bi−xxx−ate or bi−xxx−ite.
4
OR
And don’t’ forget
Hydrogen Sulfate Ion
Odd Companions or No Companion
−
about the only cation
SO
Sulfate Ion
4 2-
hydroxide OH
ammonium
NH
4 +
−
−
SO
Sulfite Ion
3 2-
cyanide
CN
thiocyanate
SCN
PO
Phosphate Ion
−
4 3-
acetate C
H
O
2
3
CrO
Chromate Ion
2-
−
2−
4
carbonate CO
bicarbonate HCO
3
3
Cr
O
Dichromate Ion
7 2-
2−
−
2
sulfate
SO
bicsulfate
HSO
4
4
OH
Hydroxide Ion
-
2-
2-
chromate CrO
dichromate Cr
O
4
2
7
C
O
Oxalate Ion
4 2-
2
−
permanganate MnO
O
Peroxide Ion
4
2-
2
−
hydroxide OH
MnO
Permangat Ion
4 -
−
oxalate C
O
SCN
Thiocyanate Ion
-
2
4
2-
2−
CN
Cyanide Ion
oxide O
peroxide
O
-
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








