Bonding Basics
ADVERTISEMENT
Name:
Period:
Bonding Basics
Essential Vocabulary:
Chemical bond –
Octet rule –
Oxidation number –
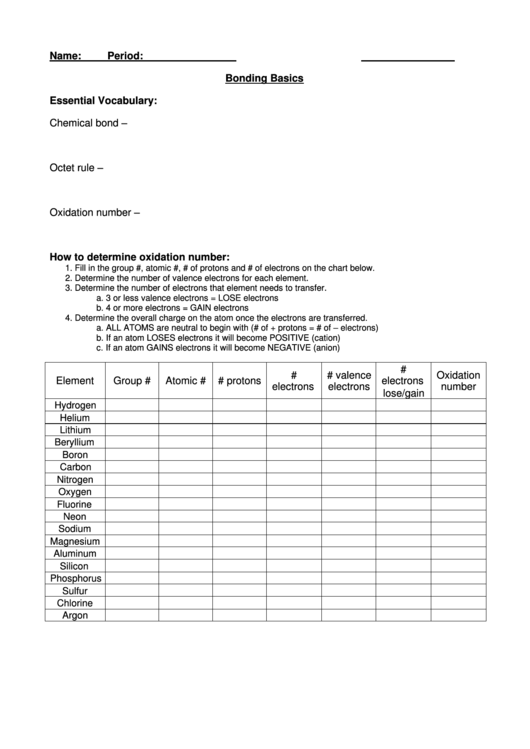
How to determine oxidation number:
1. Fill in the group #, atomic #, # of protons and # of electrons on the chart below.
2. Determine the number of valence electrons for each element.
3. Determine the number of electrons that element needs to transfer.
a. 3 or less valence electrons = LOSE electrons
b. 4 or more electrons = GAIN electrons
4. Determine the overall charge on the atom once the electrons are transferred.
a. ALL ATOMS are neutral to begin with (# of + protons = # of – electrons)
b. If an atom LOSES electrons it will become POSITIVE (cation)
c. If an atom GAINS electrons it will become NEGATIVE (anion)
#
#
# valence
Oxidation
Element
Group #
Atomic #
# protons
electrons
electrons
electrons
number
lose/gain
Hydrogen
Helium
Lithium
Beryllium
Boron
Carbon
Nitrogen
Oxygen
Fluorine
Neon
Sodium
Magnesium
Aluminum
Silicon
Phosphorus
Sulfur
Chlorine
Argon
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3