Electronegativity Questions
ADVERTISEMENT
Passage I
2. The greatest electronegativity in the table is:
F. fluorine (F).
G. chlorine (Cl).
H. rubidium (Rb).
J. hydrogen (H).
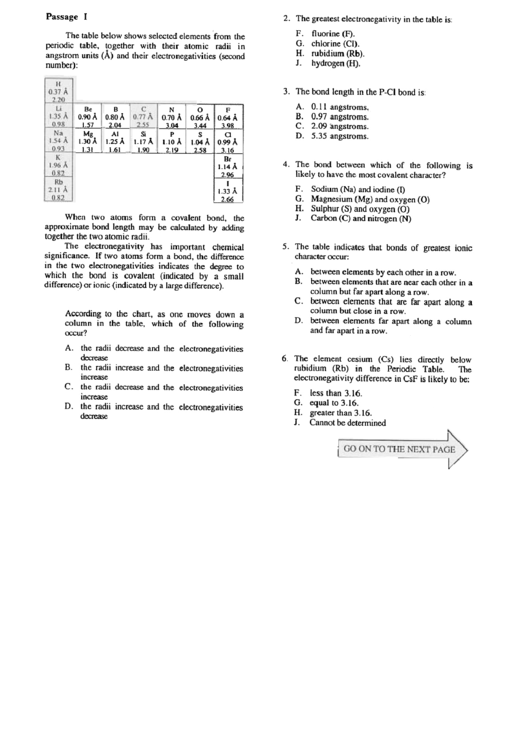
The table below shows selected elements from the
periodic table, together with their atomic radii in
angstrom units (A) and their electronegativities (second
number):
~
3. The bond length in the P-CI bond is
A. 0.11 angstroms,
B. 0.97 angstroms.
C. 2.09 angstroms.
D. 5.35 angstroms.
Be
B
0.90
A
0.80
A
1.57~
Mg
AI
1.30 A t.25 A
, 1.31 . 1.61
N
0
0.70
A
0.66
A
3.04
i
3.44
Si
P
S
1.17A
1.IOA
1.04A
1.90
2.19
2.58
F
0.64
A
3.98
~
'9~
A.
3.16
Br
1.14 A.
~I
I
1.33 A
, 2.66 ,
4. The bond between which of the following is
likely to have the most covalent character?
F. Sodium (Na) and iodine (1)
G. Magnesium (Mg) and oxygen (0)
H. Sulphur (S) and oxygen (0)
J. Carbon (C) and nitrogen (N)
When
two atoms form a covalent bond, the
approximate bond length may be calculated by adding
together the two atomic radii.
The electro negativity has important chemical
significance. If two atoms form a bond, the difference
in the two electronegativities indicates the degree to
which the bond is covalent (indicated by a small
difference) or ionic (indicated by a large difference).
According to the chart, as one moves down a
column in the table, which of the following
occur?
A. the radii decrease and the electronegativities
decrease
B. the radii increase and the electronegativities
increase
~.
the radii decrease and the electronegativities
increase
D. the radii increase and the electronegativities
decrease
5. The table indicates that bonds of greatest ionic
character occur:
A. between elements by each other in a row.
B. between elements that are near each other in a
column but far apart along a row.
C. between elements that are far apart along a
column but close in a row.
D. between elements far apart along a column
and far apart in a row.
6
r
The element cesium (Cs) lies directly below
rubidium (Rb) in the Periodic Table.
The
electronegativity difference in CsF is likely to be:
F. less than 3.16.
G. equal to 3.16.
H. greater than 3.16.
J. Cannot be determined
GO ON TO THE NEXT PAGE
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








