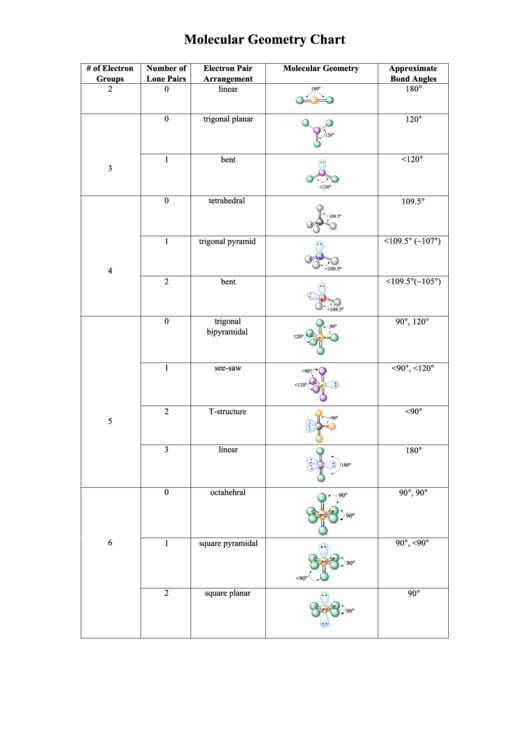
Molecular Geometry Chart
ADVERTISEMENT
Molecular Geometry Chart
# of Electron
Number of
Electron Pair
Molecular Geometry
Approximate
Groups
Lone Pairs
Arrangement
Bond Angles
2
0
linear
180°
o
180
0
trigonal planar
120°
o
120
1
bent
<120°
3
o
<120
0
tetrahedral
109.5°
o
109.5
1
trigonal pyramid
<109.5° (~107°)
o
4
<109.5
2
bent
<109.5°(~105°)
o
<109.5
0
trigonal
90°, 120°
o
90
bipyramidal
o
120
1
see-saw
<90°, <120°
o
<90
o
<120
2
T-structure
<90°
o
<90
5
3
linear
180°
o
180
0
octahehral
90°, 90°
o
90
o
90
6
1
square pyramidal
90°, <90°
o
90
o
<90
2
square planar
90°
o
90
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1