Uv-Visible Spectroscopy - Spectra
ADVERTISEMENT
C h e m g u id e – q u e s t i o n s
UV-VISIBLE SPECTROSCOPY – SPECTRA
1. When light passes through a compound some wavelengths are absorbed because their energy is
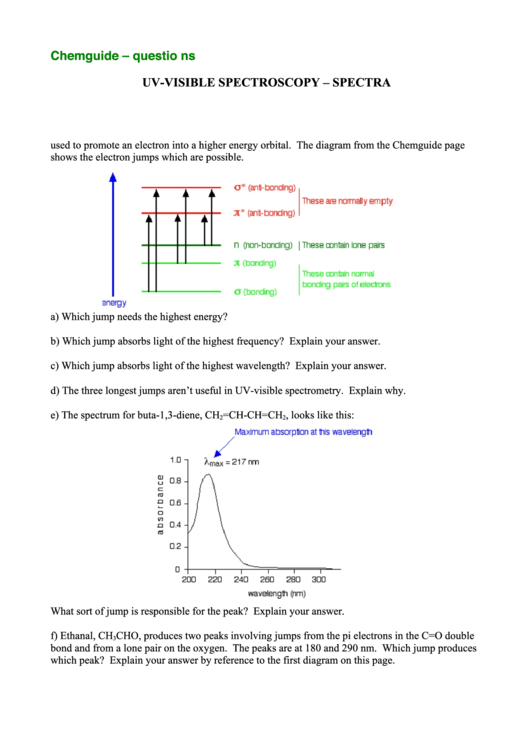
used to promote an electron into a higher energy orbital. The diagram from the Chemguide page
shows the electron jumps which are possible.
a) Which jump needs the highest energy?
b) Which jump absorbs light of the highest frequency? Explain your answer.
c) Which jump absorbs light of the highest wavelength? Explain your answer.
d) The three longest jumps aren’t useful in UV-visible spectrometry. Explain why.
e) The spectrum for buta-1,3-diene, CH
=CH-CH=CH
, looks like this:
2
2
What sort of jump is responsible for the peak? Explain your answer.
f) Ethanal, CH
CHO, produces two peaks involving jumps from the pi electrons in the C=O double
3
bond and from a lone pair on the oxygen. The peaks are at 180 and 290 nm. Which jump produces
which peak? Explain your answer by reference to the first diagram on this page.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3