Change Of State Problems Chemistry I Honours
ADVERTISEMENT
Chemistry I-Honors
Change-of-State Problems – Solution Set
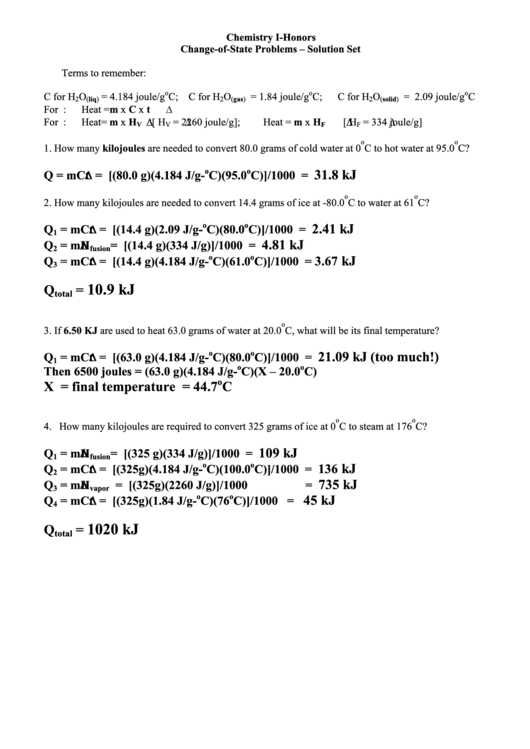
Terms to remember:
o
o
o
C for H
O
= 4.184 joule/g
C; C for H
O
= 1.84 joule/g
C;
C for H
O
= 2.09 joule/g
C
2
(liq)
2
(gas)
2
(solid)
For K.E.:
Heat = m x C x t
For P.E.:
Heat = m x H
[ H
= 2260 joule/g];
Heat = m x H
[ H
= 334 joule/g]
V
V
F
F
o
o
1. How many kilojoules are needed to convert 80.0 grams of cold water at 0
C to hot water at 95.0
C?
o
o
31.8 kJ
Q = mC t = [(80.0 g)(4.184 J/g-
C)(95.0
C)]/1000 =
o
o
2. How many kilojoules are needed to convert 14.4 grams of ice at -80.0
C to water at 61
C?
o
o
2.41 kJ
Q
= mC t = [(14.4 g)(2.09 J/g-
C)(80.0
C)]/1000 =
1
4.81 kJ
Q
= m H
= [(14.4 g)(334 J/g)]/1000
=
2
fusion
o
o
kJ
3.67
Q
= mC t = [(14.4 g)(4.184 J/g-
C)(61.0
C)]/1000 =
3
10.9 kJ
Q
=
total
o
3. If 6.50 KJ are used to heat 63.0 grams of water at 20.0
C, what will be its final temperature?
o
o
21.09 kJ (too much!)
Q
= mC t = [(63.0 g)(4.184 J/g-
C)(80.0
C)]/1000 =
1
o
o
Then 6500 joules = (63.0 g)(4.184 J/g-
C)(X – 20.0
C)
o
X = final temperature = 44.7
C
o
o
4. How many kilojoules are required to convert 325 grams of ice at 0
C to steam at 176
C?
109 kJ
Q
= m H
= [(325 g)(334 J/g)]/1000
=
1
fusion
o
o
kJ
136
Q
= mC t = [(325g)(4.184 J/g-
C)(100.0
C)]/1000 =
2
735 kJ
Q
= m H
= [(325g)(2260 J/g)]/1000
=
3
vapor
o
o
45 kJ
Q
= mC t = [(325g)(1.84 J/g-
C)(76
C)]/1000
=
4
1020 kJ
Q
=
total
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








