Revision Guide Alkenes 3.4 - Chemistry Sheet Page 2
ADVERTISEMENT
Electrophilic Addition Reactions of Alkenes
Definition Electrophile: an electron pair acceptor
The double bonds in alkenes are areas with high
electron density. This attracts electrophiles and the
Addition reaction: a reaction where two
alkenes undergo addition reactions.
molecules react together to produce one
1. Reaction of Bromine with Alkenes
H
H
Change in functional group: alkene
dihalogenoalkane
H
H
Reagent: Bromine
H
C
C
H
+ Br
C
C
Conditions: Room temperature (not in UV light)
2
Mechanism: Electrophilic Addition
H
H
Br
Br
δ+
Type of reagent: Electrophile, Br
1,2-dibromoethane
H
H
As the Br
molecule
H
H
H
H
2
approaches the alkene,
The INTERMEDIATE
+
the pi bond electrons
C
C
H
C
C
H
H
C
C
H
formed, which has a
repel the electron pair in
positive charge on a
the Br-Br bond. This
Br
H
H
Br
carbon atom is called a
Br
Br
INDUCES a DIPOLE. Br
+
δ
2
CARBOCATION
-
:Br
becomes polar and
Br
δ+
ELECTROPHILIC (Br
).
-
δ
2. Reaction of Hydrogen Bromide with Alkenes
Change in functional group: alkene halogenoalkane
H
H
H
H
H
H
Reagent: HCl or HBr
H
C
C
C
C
H
+ HBr
H
C
C
C
C
H
Conditions: Room temperature
Mechanism: Electrophilic Addition
H
H
H
H
H
H
Br
H
δ+
Type of reagent: Electrophile, H
But-2-ene
2-bromobutane
HBr is a polar
H
H
H
H
This reaction can
H
molecule because
H
lead to two
Br is more
H
C
C
C
CH
+
H
C
C
C
CH
products when the
3
3
H
C
C
C
CH
electronegative
3
3
3
3
alkene is
+
δ
δ +
than H. The H
is
H
H
H
unsymmetrical
Br
attracted to the
-
electron-rich pi
δ
-
Br
:Br
bond.
‘Markownikoff’s Rule’
-
:Br
H
In most cases, bromine will be added to the
H
H
H
H
+
carbon with the fewest hydrogens attached to it
C
CH
2
Major
H
C
C
C
C
H
product
If the alkene is
H
C
CH
3
3
90%
unsymmetrical,
+
-
δ
δ
-
:Br
H
H
Br
H
H
Br
addition of hydrogen
H
H
bromide can lead to
Minor
+
two isomeric
CH
CH
CH
CH
C
C
CH
CH
2
H
C
CH
CH
CH
2
2
3
2
3
2
2
3
product
products.
Br
H
10%
H
WHY?
In electrophilic addition to alkenes, the major product is
This carbocation intermediate
formed via the more stable carbocation intermediate.
H
H
H
is more stable because the
methyl groups on either side
In exam answers
H
C
C
C
H
+
of the positive carbon are
•Draw out both carbocations and identify as primary, secondary
electron releasing and reduce
and tertiary
H
H
the charge on the ion which
•State which is the more stable carbocation e.g. secondary more
stabilises it.
stable than primary
•State that the more stable carbocation is stabilised because the
The order of stability for carbocations is
methyl groups on either (or one) side of the positive carbon are
electron releasing and reduce the charge on the ion.
tertiary > secondary >primary
•(If both carbocations are secondary then both will be equally
2
stable and a 50/50 split will be achieved)
N Goalby
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1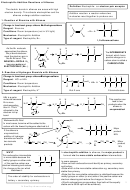 2
2 3
3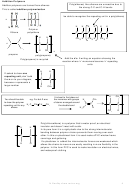 4
4