Revision Guide Alkenes 3.4 - Chemistry Sheet Page 4
ADVERTISEMENT
Addition Polymers
Poly(alkenes) like alkanes are unreactive due to
Addition polymers are formed from alkenes
the strong C-C and C-H bonds
This is called addition polymerisation
be able to recognise the repeating unit in a poly(alkene)
n
H
H
H
H
H
H
Monomer
Polymer
Ethene
polyethene
C
C
C
C
C
C
H
H
H
H
CH
H
CH
H
CH
H
3
3
3
n
C
C
C
C
H
CH
3
H
CH
n
3
propene
poly(propene)
Add the n’s if writing an equation showing the
Poly(propene) is recycled
reaction where ‘n’ monomers become ‘n’ repeating
units
H
H
If asked to draw one
repeating unit, don’t add
C
C
the n on to your diagram,
because n represents a
large number
H
CH
3
H
CH
It is best to first draw out
3
H
CH
the monomer with groups
You should be able
e.g. For but-2-ene
3
of atoms arranged around
to draw the polymer
C
C
C
C
H
C
CH CH
CH
the double bond
repeating unit for any
3
3
H
H
C
alkene
3
CH
H
3
Poly(chloroethene) is a polymer that is water proof, an electrical
insulator and doesn’t react with acids.
H
H
In its pure form it is a rigid plastic due to the strong intermolecular
bonding between polymer chains prevents them moving over each
C
C
other. In this un-plasticised form it is used make uPVC window frame
coverings and guttering.
If a plasticiser is added the intermolecular forces are weakened which
H
Cl
allows the chains to move more easily resulting in more flexibility in the
polymer. In this form PVC is used to make insulation on electrical wires,
and waterproof clothing
N Goalby
4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1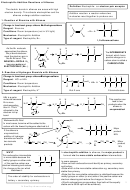 2
2 3
3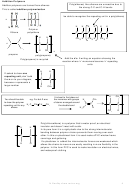 4
4